The Latest
-
Over 1,000 HHS employees call for RFK Jr. to step down
The letter cites turmoil at the CDC, which is reeling from the firing of Director Susan Monarez and the departure of at least four high-level leaders last week.
-
Medtronic advances Abbott partnership with FDA nod
The company received FDA authorization to pair its 780G insulin pump with a glucose sensor made by Abbott, and to use the device for Type 2 diabetes.
-
3 medtech takeaways from ESC 2025
Pulsed field ablation and transcatheter aortic valve implants were in focus at the European Society of Cardiology Congress in Madrid.
-
Edwards, Medtronic and Abbott stand to benefit from new TAVR guidelines in Europe
Analysts said the companies should benefit “as the therapy algorithm is further streamlined and additional patients are encouraged to receive TAVR.”
-
AI devices with no clinical validation tied to more recalls, study finds
Public companies, which accounted for about half of AI-enabled devices on the market, had a higher rate of recalls and a lower rate of clinical evidence, according to a JAMA study.
-
Medtech VC funding on track to hit highest value since 2021: PitchBook
“Larger rounds increasingly favor top-tier companies and AI-native startups, leaving other startups fighting for a smaller pool of capital,” the firm said.
-
Appellate court rules Trump’s global tariffs illegal, but delays action
The court intends to strike down the tariffs by mid-October, pending a Supreme Court review of the ruling.
-
Deep Dive
4 medtech topics to watch for the rest of 2025
From M&A to MDUFA and the developing market in renal denervation, MedTech Dive covers the key issues to watch in the final months of the year.
-
Intuitive to lay off 331 employees in California
The robotic surgery company, which filed a WARN notice with the state in August, plans for the layoffs to be effective in late October.
Updated Aug. 28, 2025 -
J&J removes some Abiomed heart pump controllers from market
Abiomed reported one death associated with a pump driver circuit assembly that does not meet current specifications. The recall affects 69 controllers, a J&J spokesperson said.
-
Inspire CFO Rick Buchholz to step down at end of 2025
RBC analysts said their views on the company are unchanged because the departing CFO will see the “Inspire V roll-out efforts through to completion.”
-
Deep Dive
White House data sharing plan boasts big ambitions, but has scant details
Improving health data exchange is a worthy goal, but the initiative has to overcome challenges like data security, under-resourced providers and slow technology uptake, experts say.
-
Quest forms joint venture with Corewell Health
The diagnostics company will open a lab with Corewell Health in 2027 and manage the not-for-profit health system’s existing lab facilities.
-
Insulet promotes Eric Benjamin to chief operating officer
As new CEO Ashley McEvoy works to build the brand globally, the diabetes tech company also hired Manoj Raghunandanan as chief growth officer.
-
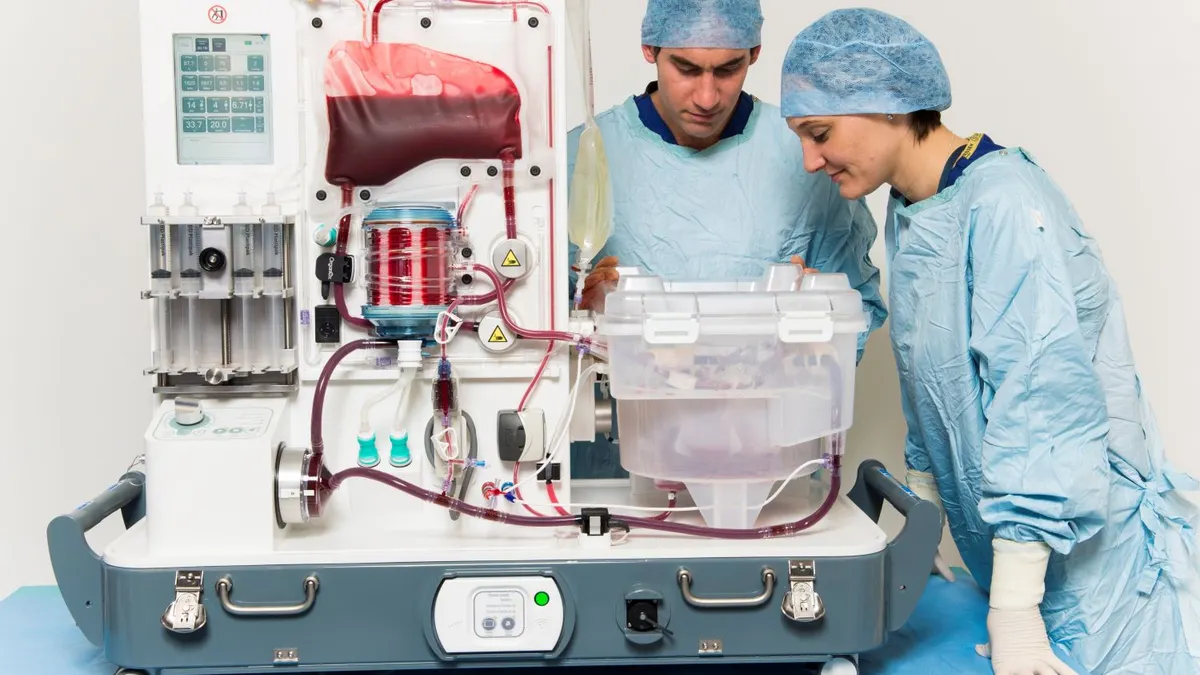
Terumo to buy OrganOx for $1.5B to enter transplant field
OrganOx could capture a greater share of the liver transplant market, where it competes with TransMedics Group, Needham analyst Mike Matson wrote.
-
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
-
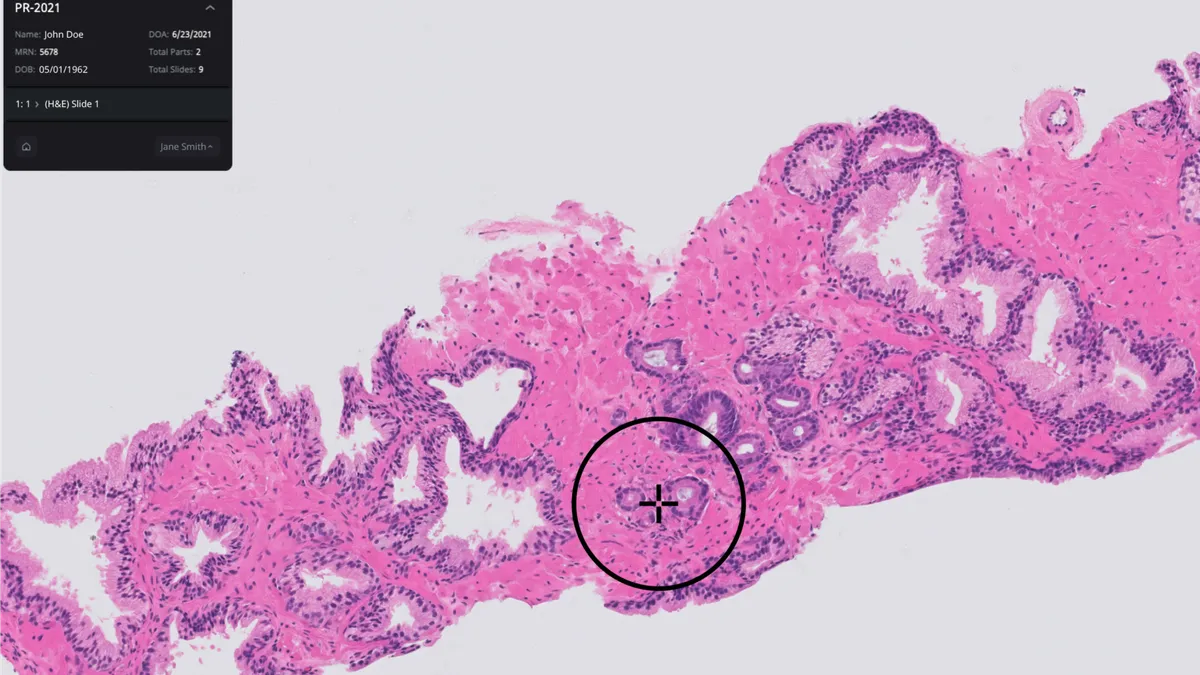
Tempus inks $81M Paige buyout to support AI model development
CEO Eric Lefkofsky said that buying Paige “substantially accelerates” Tempus’ effort to build the largest foundation model in oncology.
-
Medtech firms shuffle execs in 2025
Top leaders at Intuitive, Boston Scientific, Dexcom and others changed roles this year. Read MedTech Dive’s roundup for all the details.
-
MedTech Europe sounds alarm over impact of US-EU tariffs on patients
The trade group wants the U.S. to eliminate tariffs on medical technologies, warning that “patients cannot be collateral damage in trade tensions.”