Page 2
-
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
-
Tandem, Insulet monitoring CMS payment proposal for diabetes tech
The proposal would introduce competitive bidding for insulin pumps and change how Medicare pays for diabetes devices, if finalized.
-
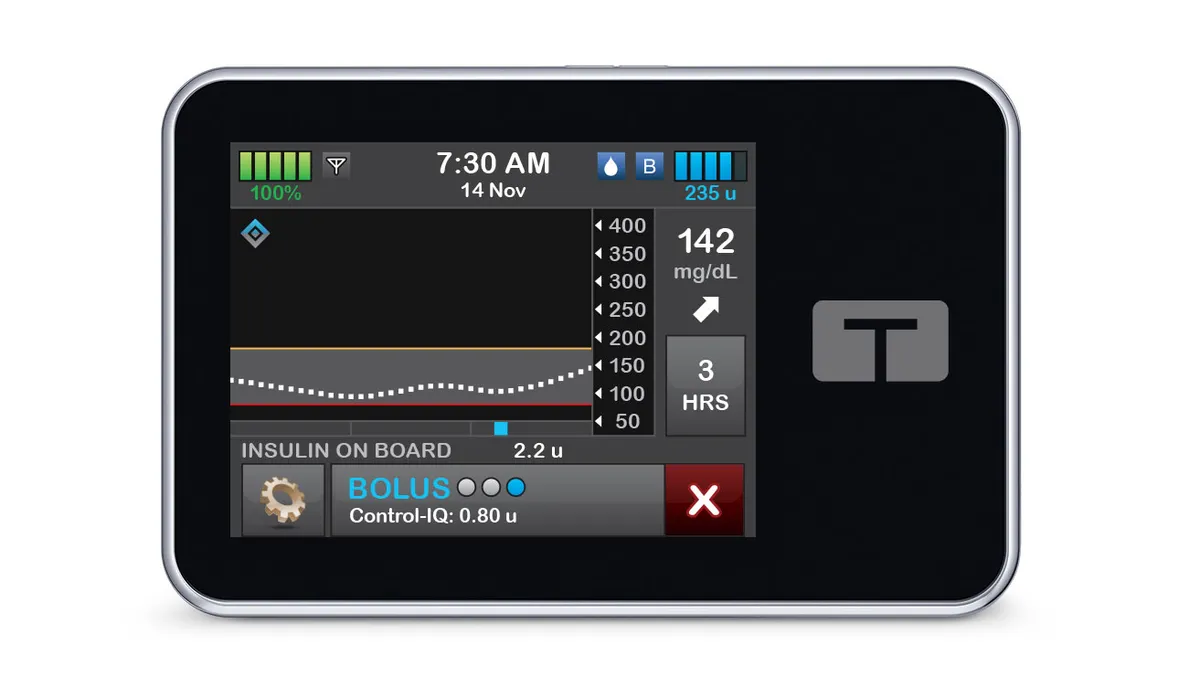
Tandem insulin pump malfunction linked to 59 injuries
A problem with speakers in Tandem’s t:slim X2 insulin pumps can cause insulin delivery to stop, the company said.
-
Masimo appoints several senior execs, resolves cyber incident
The pulse oximeter maker is now fully operational after a spring cyberattack and increased its 2025 profit forecast, but investors have raised questions about the status of a partnership with Philips.
-
Boston Scientific tells users about defibrillator problem linked to deaths, injuries
A company spokesperson said in a statement to MedTech Dive that direct causation between the deaths and calcification of the leads cannot be assumed or confirmed.
-
FTC moves to block Edwards’ JenaValve acquisition
Edwards Lifesciences and JenaValve said they remained committed to completing the deal and would defend the case in court.
-
Zimmer is latest medtech firm to lower expected tariff hit
CFO Suketu Upadhyay said the company now expects a charge in 2025 of about $40 million, compared with a range of $60 million to $80 million.
-
Trump plans 100% tariffs for semiconductor imports
Chips are one of several product types currently under Section 232 investigation by the White House, a frequent precursor to duties.
-
BD commits $35M to expand US syringe production plant
The company said it has grown capacity by 10% this year, bringing its total U.S. output of the prefilled flush syringes to above 750 million units.
-
Medical device industry says future MDUFA hikes unsustainable
Device lobbyists’ priorities contrasted with patient advocates, who sought an increase in user fees and more funds for postmarket safety.
-
Alcon to acquire STAAR Surgical for about $1.5B
BTIG analyst Ryan Zimmerman called the transaction “a solid deal” for Alcon given STAAR’s struggles in the China market.
-
Philips BiPAP machine recall associated with 8 deaths
Philips sent updated instructions after disclosing problems last year with an alarm that can interrupt treatment for people with obstructive sleep apnea or respiratory insufficiency.
-
EU delays countermeasures to US tariffs by 6 months
The bloc has now paused implementation three times, with the latest moratorium coming after reaching a framework deal with the U.S.
-
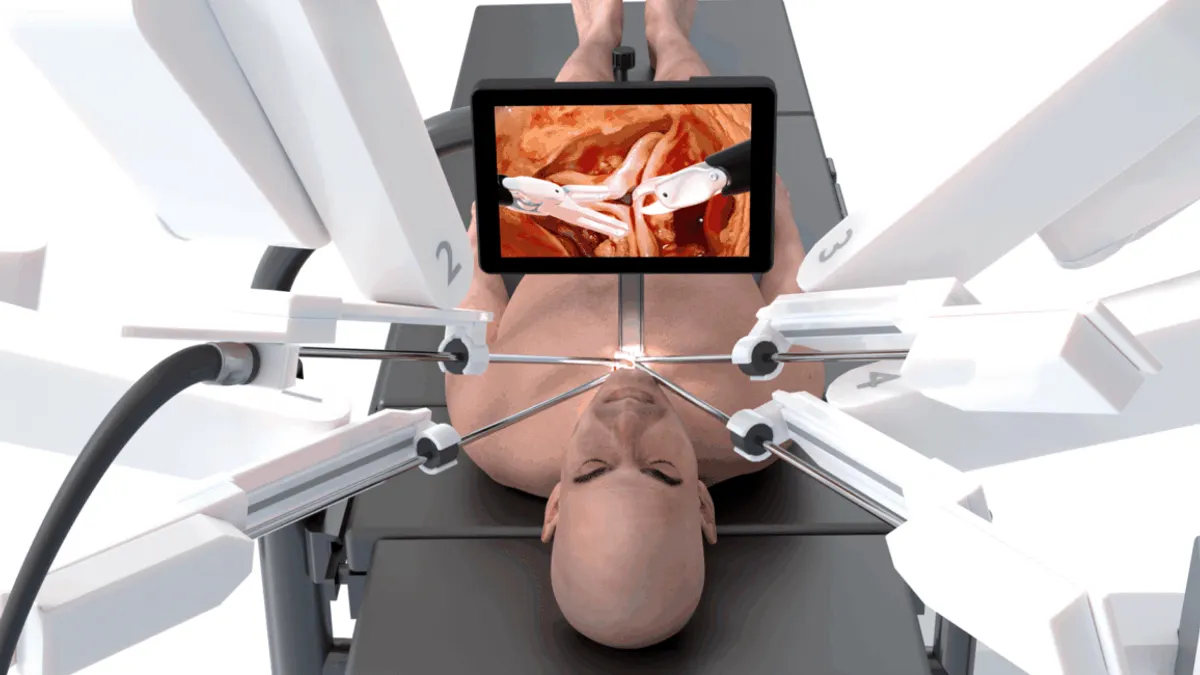
First Corcym aortic valve implanted via new robotic procedure
CEO Christian Mazzi said the minimally invasive surgical technique could provide better outcomes for patients than transcatheter aortic valve replacement.
-
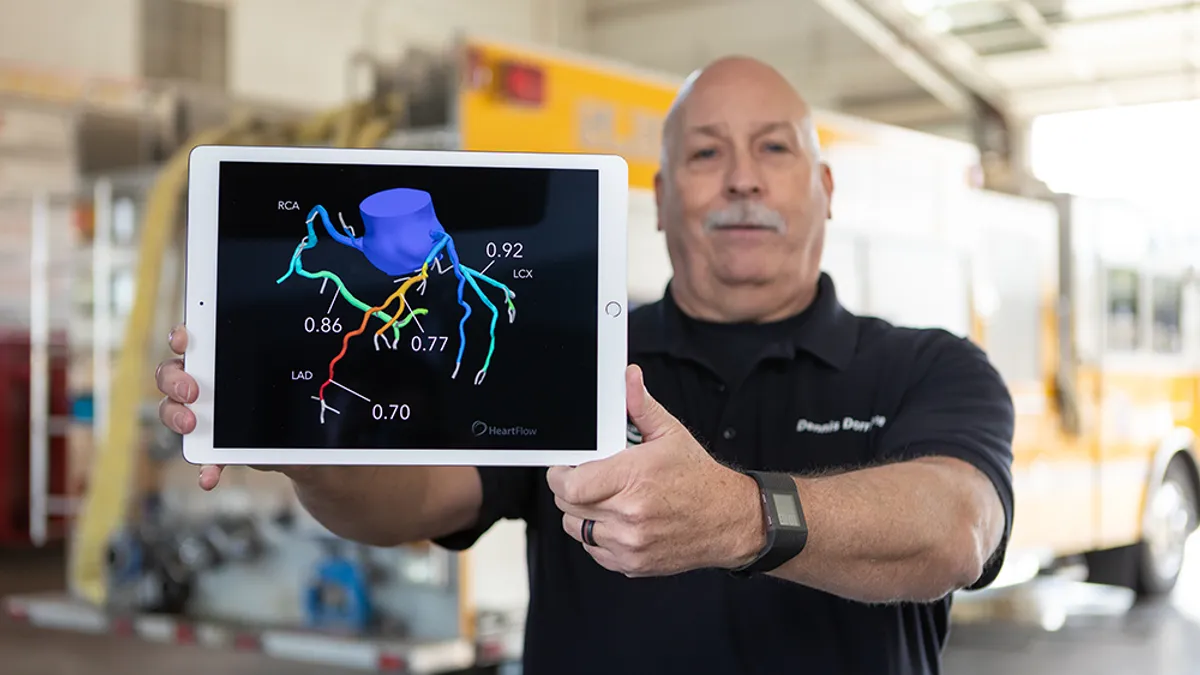
Heartflow prices IPO, aiming to net about $180M
The company, which sells software for creating 3D heart models, plans to offer 12.5 million shares at a range of $15 to $17 per share.
-
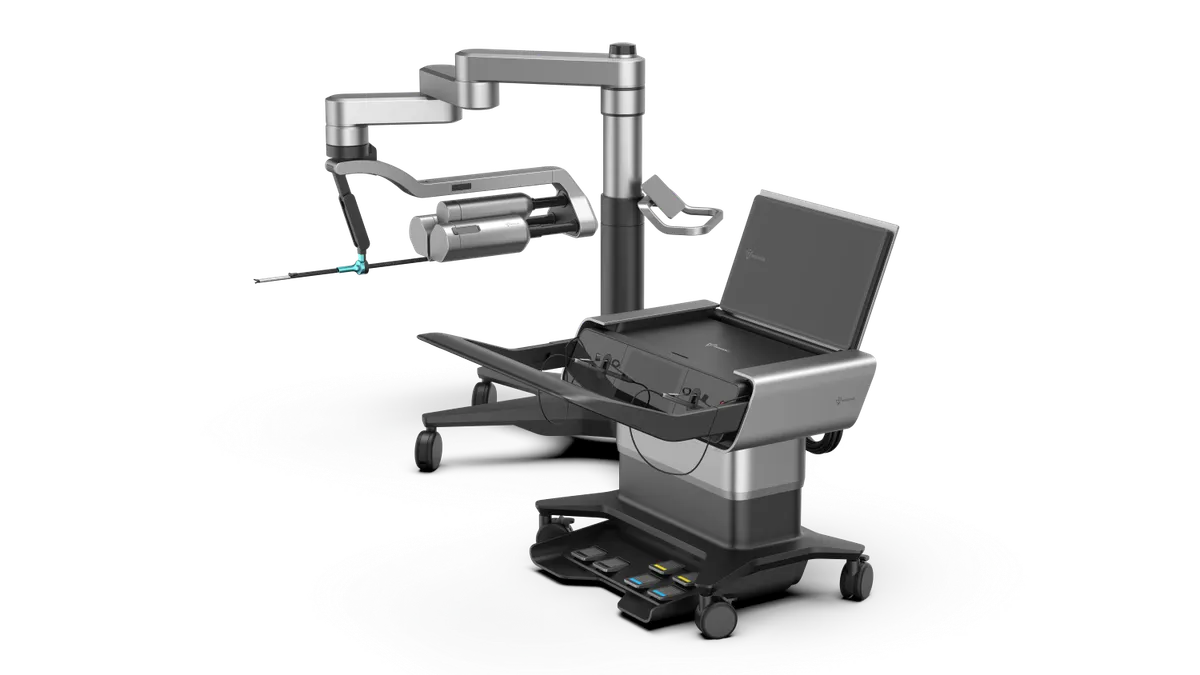
Vicarious Surgical names Stephen From as next CEO
From, who joins the surgical robot developer from Aruna Bio, will take the reins from co-founder Adam Sachs on Aug. 7.