Dive Brief:
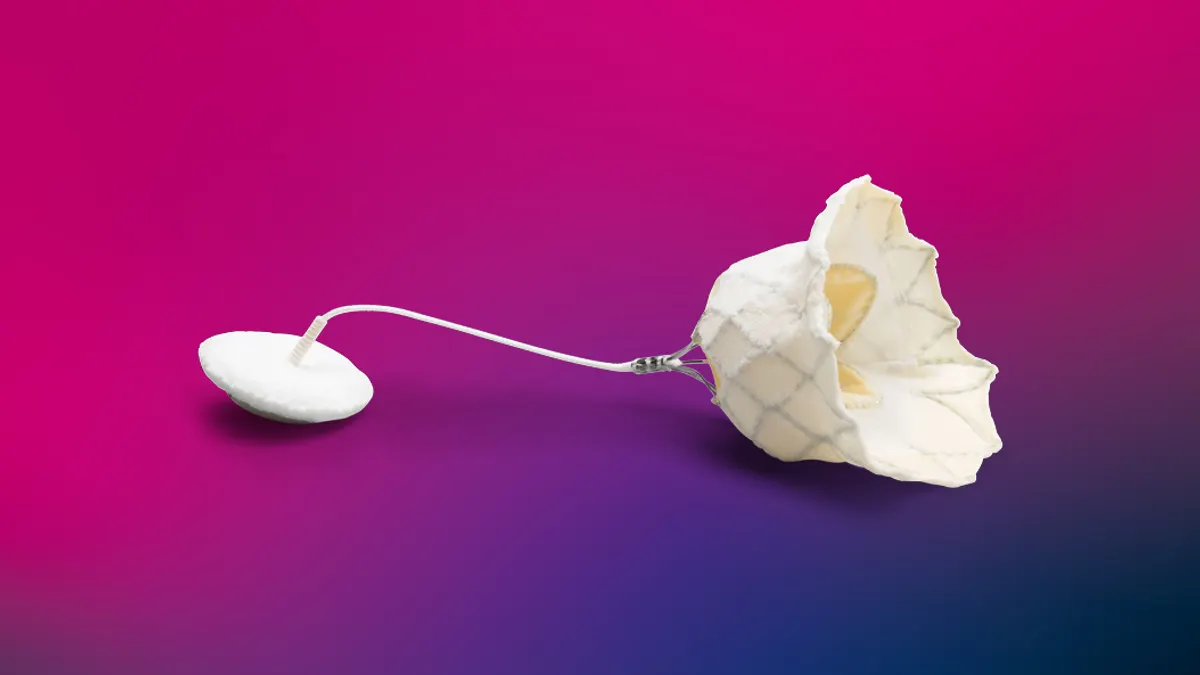
- Abbott said Tuesday it received Food and Drug Administration approval for the Tendyne transcatheter mitral valve replacement system to treat calcium buildup in the ring that supports the heart valve.
- The device is available for patients with severe mitral annular calcification who are not candidates for open heart surgery or transcatheter mitral valve repair.
- Abbott’s MitraClip system for mitral valve repair competes with Edwards Lifesciences’ Pascal repair device. The rivals are now set to compete in mitral valve replacement: Edwards won Europe’s CE mark last month for the Sapien M3 transfemoral system and expects U.S. approval in 2026.
Dive Insight:
Abbott acquired Tendyne for $250 million in September 2015 and received CE mark approval for the system in January 2020.
“The approval culminates almost a decade of effort by Abbott,” Citi Research analyst Joanne Wuensch said in a report Tuesday, noting the commercialization of new valve technologies expands the options for patients.
Severe mitral annular calcification prevents the heart from pumping blood effectively. Abbott said the Tendyne system addresses a significant unmet need in cardiac care, offering a minimally invasive way to replace the mitral valve for patients whose valves cannot be successfully repaired with MitraClip.
"Patients with MAC can be very difficult to operate on and many are considered too high risk for open-heart surgery due to multiple co-morbidities or other factors. Tendyne bridges a critical treatment gap for these patients,” Paul Sorajja, director of the Center for Valve and Structural Heart Disease at the Minneapolis Heart Institute at Abbott Northwestern Hospital, said in Abbott’s statement.
The self-expanding Tendyne valve is delivered to the heart through a small incision in the chest and is available in multiple sizes for a range of patient anatomies.