Christopher Piorkowski, chief medical officer of Abbott’s large and fast-growing electrophysiology business, recently visited clinical sites in Australia and across Europe to gather feedback on the first patients enrolled in a pre-market study of the company’s Volt pulsed field ablation (PFA) system.
PFA is a new technique for treating atrial fibrillation (AFib), a heart rhythm disorder that increases a patient’s risk for stroke from a blood clot. The treatment is attracting considerable investment from medtech companies: Medtronic in December became the first device maker to gain Food and Drug Administration approval for a PFA system, and Boston Scientific and Johnson & Johnson are also pursuing the market.
Abbott expects approval for a U.S. clinical trial to study the Volt system in the first half of this year. While other device makers are further along with their PFA systems, Abbott is working to address limitations it sees in the first generation of devices, according to Piorkowski, who explained the company’s approach in a conversation with MedTech Dive.
This interview has been edited for length and clarity.
MEDTECH DIVE: What is driving the enthusiasm for pulsed field ablation?
CHRISTOPHER PIORKOWSKI: Catheter ablation of atrial fibrillation has matured over almost 15 years now, from something that was done in very few, selected patients, at certain risks, and with a lot of scientific learning. It has progressed well, to a point that today we understand that catheter ablation of atrial fibrillation is superior to anti-arrhythmic medication, or to just leaving the patient in atrial fibrillation. It even affects a better outcome in mortality, especially when you look into patients with heart failure and atrial fibrillation.
However, the therapy itself is still something which needs a lot of training, skill, talent and time. When I look into the field of clinical medicine in my position as chief medical officer, I see a huge variability in procedure duration, outcome and safety.
What everybody was excited about when PFA came out was the promise that this is something that can be fast. It could reduce procedure time; it could standardize the procedure. It would allow more patients to be treated, because everybody has a waiting list. The other aspect was the safety profile. There is one complication that every electrophysiologist is afraid of during atrial fibrillation ablation and that's injury to the esophagus, which sits right in the zone of the ablation target. Although it is rare, it is a highly lethal complication. PFA had the promise of being tissue-selective, of not causing risk to the esophagus, and that, in my perspective, was the second main aspect that helped to build the level of expectations.
Do you expect PFA to replace radiofrequency ablation or cryoablation?
What we are seeing in Europe today, where PFA is commercially available, we see physicians switching from cryo to PFA, because cryo was also designed as a tool for the pulmonary veins. And now, this is often replaced by PFA because PFA is faster. And all these hospitals, these physicians, they have long waiting lists. They cannot treat the patients as fast as they would actually need to, so they look for something that makes their work easier. And that's the promise of PFA which holds true.
Now for radiofrequency, the story's a little bit different, because radiofrequency is quite often used outside the pulmonary veins if additional ablation targets are being addressed. When we look into the future it's always hard to predict, but I would think that there will be scenarios where the need for radiofrequency will remain.
To give a clinical example where this may be needed, we have learned over the past years that PFA can cause coronary spasms. The coronary arteries cramp and then not enough blood goes to the myocardial tissue, which can be dangerous to patients. There is now increasing consensus that if ablation is done in proximity to a coronary artery, maybe RF is the better energy source. In other areas where this proximity is not happening, PFA may be preferred. There are use cases which lead into one or the other energy source.
What does the clinical data tell us about PFA?
First, there's the hype and the expectations, and then you see reality come in. One study to look into is the Advent trial that was presented last summer, which was a comparison of a competitor's PFA technology against existing ablation technologies such as radiofrequency and cryoablation. This study basically showed, yes, PFA is feasible to treat patients. It's maybe a little faster, but the procedure durations were not so strikingly short. It is certainly something that is not more effective. The efficacy rates, rather, tend to be lower within this trial. And, yes, it's safer at the esophagus, but it's also not free from risks.
In the end, with the first-generation PFA tools that are available now, it is a mixed picture, right?
The promise that this is going to be a breakthrough that everybody can treat every patient very safe, very fast and very effective has not come true so far. In fact, there are other aspects that the field is learning with a new energy source: new risks, new side effects, new complications that need to be addressed. And therefore I would say the journey of PFA is an evolutionary journey, and I would also say that we in Abbott have known some of that quite early because we spent a lot of time and resources to study this energy source. We hoped that we could address some of the limitations that are there with first-generation devices.
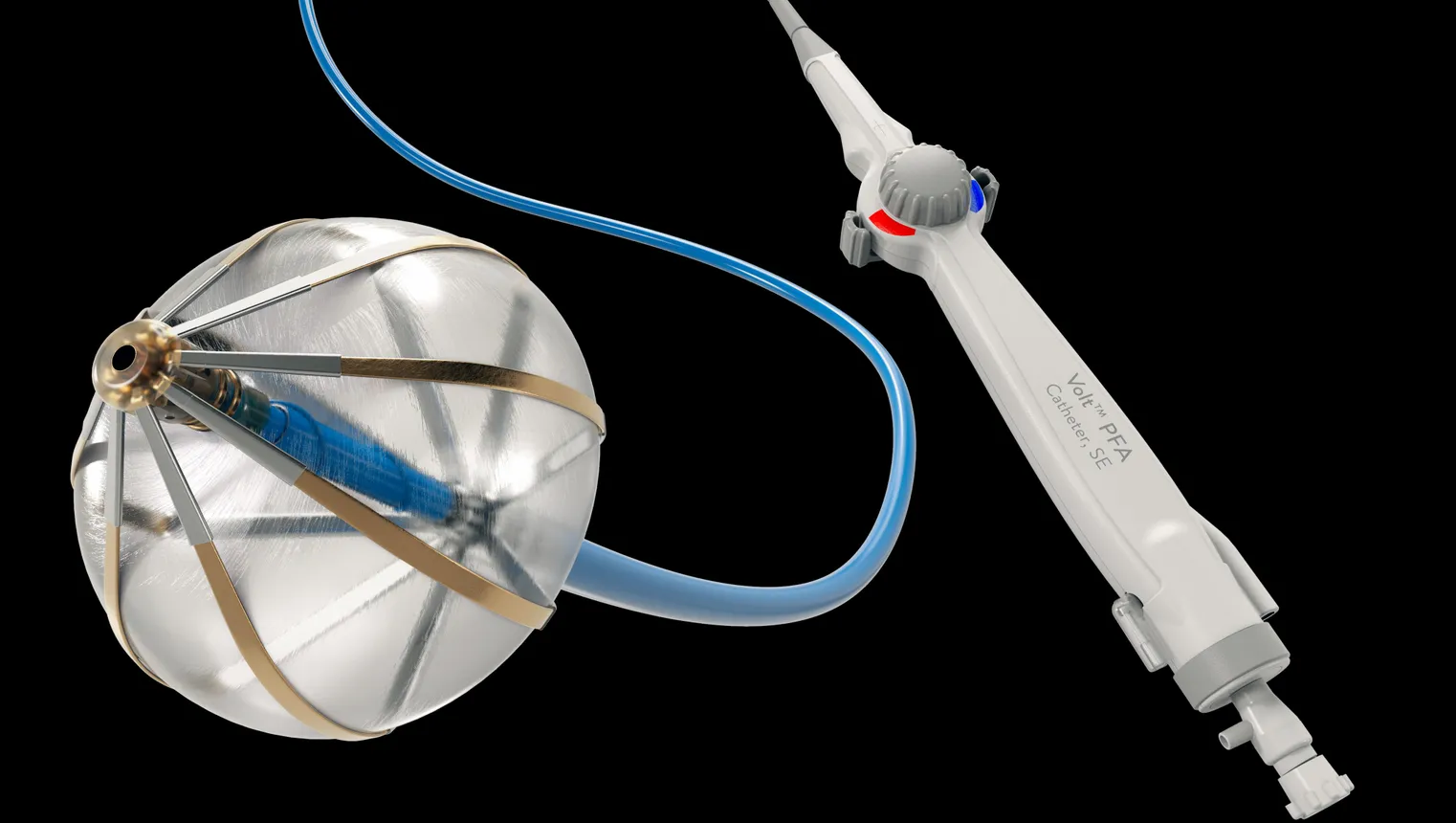
How will Volt differ from other PFA systems?
When we studied PFA, we acknowledged the benefits of a potentially very safe and very fast ablation technology. We also saw limitations in the lesions that are being delivered in terms of lesion depths and lesion durability, and that is a theme that you can find when you look through the publications of all existing PFA technologies. There is a significant amount of lesion recovery. The physician delivers the ablation lesion, he thinks the area in the heart is effectively treated, and two or three months later, he finds out that the lesion has recovered and the patient needs to be treated again.
That is something we did not want to accept in our product. We wanted to guarantee a single procedure which is highly effective. We worked a lot to improve the lesion performance of our PFA technology, and the key determinant that helped us to do this is a balloon-in-the-basket design. We have a balloon that sits in a basket of eight splines, and due to this design, the PFA energy is directed into the tissue. Without the balloon, the energy would be lost in the blood pool and would not contribute to the lesion. The balloon is an insulator of the blood pool.
The lesions that this device delivers are significantly deeper and more chronically durable than anything that is out there in PFA technology. That has a very important clinical effect. Physicians can be sure that with very few applications, the pulmonary vein is isolated. So far, we believe that we have met that expectation and that this is holding true in clinical practice.