Dive Brief:
- UroMems has begun a first-in-human clinical trial of its smart automated artificial urinary sphincter.
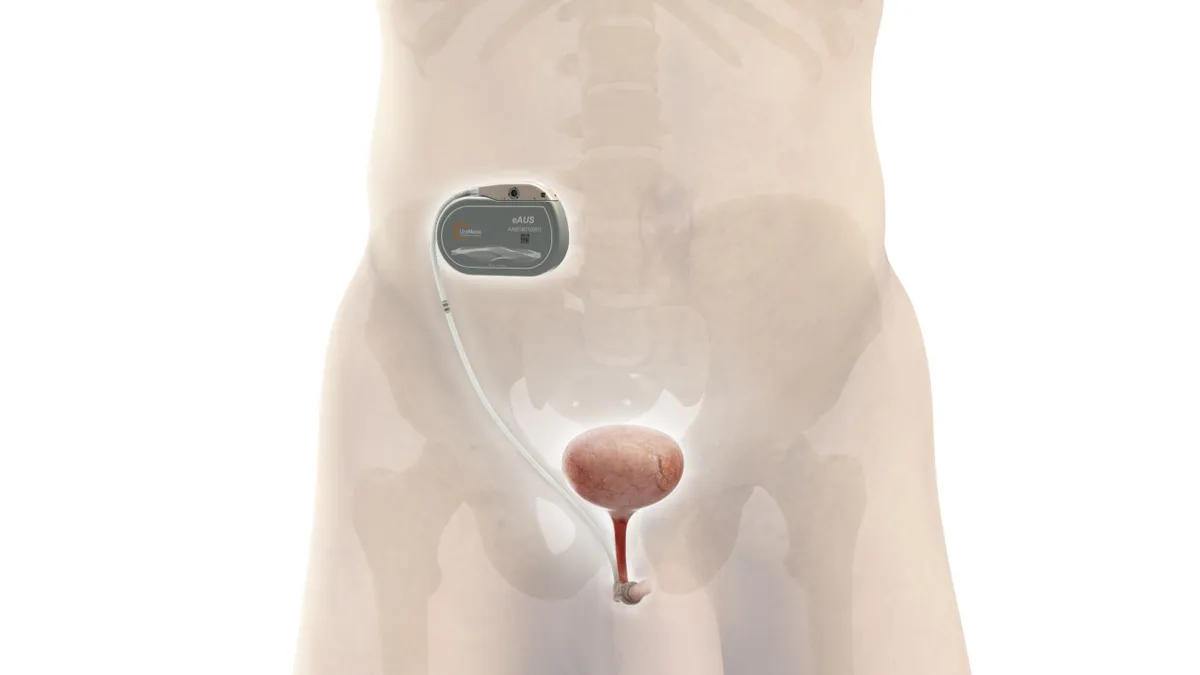
- The device, UroActive, is designed to improve on the AUSs used to treat stress urinary incontinence by eliminating the need for manual adjustments. UroActive is placed around the urethral duct and automatically controlled based on the patient’s activity.
- A physician in France performed the first procedure with UroActive, advancing UroMems toward the generation of clinical data that will start to show if the device works as intended.
Dive Insight:
Physicians have used devices such as Boston Scientific’s AMS 800 to treat SUI for decades. The devices mimic the function of a healthy sphincter to stop involuntary urinary leakage. However, patients need to operate a pump to control the device, something that is beyond the dexterity of some individuals, and constant pressure can lead to the recurrence of incontinence.
Researchers at institutions including VA Medical Center and Polystim Neurotechnologies Laboratory have worked on smart AUS devices for years, seeking to replace manual pumps with sensor-enabled systems that automate urinary control.
UroMems is working to bring such a technology to market. The implant features sensors that measure the patient’s activity. Algorithms interpret the sensor data and tell the device how much pressure to apply to the urethra. The device could change the level of pressure applied in response to the activity of the patient, for example by reducing it when they are lying down and increasing it during exercise.
A male patient now has the UroActive device implanted. The procedure took place at a hospital in France as part of a clinical trial that UroMems is running to support its push to bring the technology to market.
“This is the next important step in delivering our novel technology to a large, underserved patient population with unmet needs not addressed by current options on the market,” UroMems CEO Hamid Lamraoui said in a statement.