Five years ago, Medtronic executives set out to change the company culture and prioritize financial performance alongside innovation.
The medtech giant centralized operations to lower costs, brought in new leaders and added performance incentives to hasten the culture shift. Capital and human resources were allocated to areas of medtech with the highest growth potential.
On Monday, CEO Geoff Martha told investors the company’s product pipeline is now well-positioned to drive improved earnings.
Medtronic's biggest businesses, which include cardiac rhythm management, spine and transcatheter heart valves, are “growing well,” the CEO said, “and then we're stacking growth drivers on top of growth drivers” in fast-developing medtech markets.
Speaking at the J.P. Morgan Healthcare Conference in San Francisco, Medtronic executives outlined four categories where the company expects the highest growth from new products in the near term: cardiac ablation, renal denervation, neuromodulation and automated insulin delivery systems for diabetes management.
Scaling up PFA
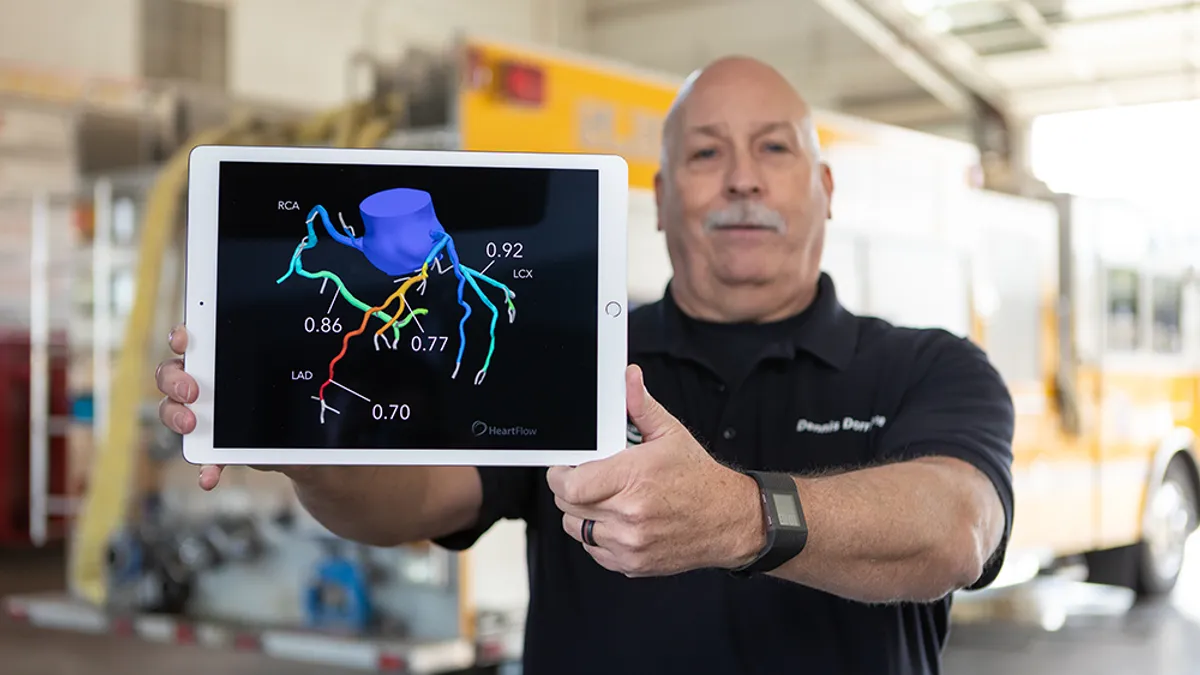
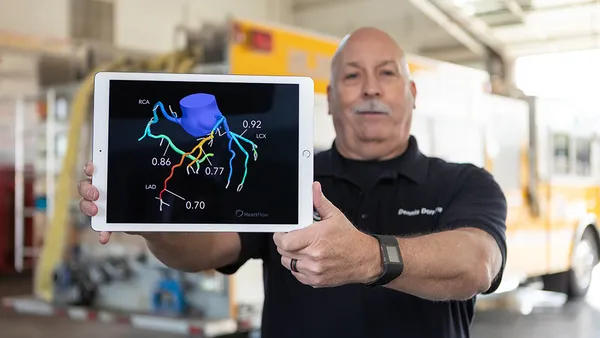
Medtronic is racing against Boston Scientific to expand in one of medtech’s hottest markets, pulsed field ablation (PFA). The new technology treats atrial fibrillation, an abnormal heartbeat that can lead to blood clots and stroke.
Medtronic is adding manufacturing capacity in Galway, Ireland, to meet demand, said Sean Salmon, president of cardiovascular.
The company’s PulseSelect PFA catheter is scaling up, while the recently launched Affera dual-energy PFA and radiofrequency system with mapping technology has met with “huge enthusiasm” from physicians as the company targets high-volume electrophysiology centers, Salmon said.
Martha pegged the cardiac ablation market at $9 billion and predicted “strong double-digit growth” for Medtronic’s business in the company’s fiscal third quarter.
Johnson & Johnson, the third PFA competitor to reach the U.S. market, last week temporarily halted patient cases in the U.S. to investigate a safety concern. Touting the clinical data for both PulseSelect and Affera, Martha said, “As we've seen over the last week, safety here really matters.”
RDN gets coverage analysis
Martha called the Centers for Medicare and Medicaid Services’ decision to open a national Medicare coverage analysis for renal denervation a “pivotal development” in expanding access to the new treatment for high blood pressure.
Medtronic has made progress over several years getting Medicare coding and payment systems in place for the Symplicity Spyral device as it prepares to address the “massive” hypertension market, Martha said.
The global hypertension market is just 1% penetrated, and that percentage alone represents a $1 billion opportunity, said Martha. The CEO noted that half of all heart disease and stroke-related deaths are caused by the condition. “If you have hypertension, it’s likely that you don’t have it under control,” he added.
Renal denervation “is a safe, one-time, very tolerable procedure,” said Martha. “This is going to change the game.”
Expanding in neuromodulation with BCI
Medtronic’s neuromodulation business is gaining new momentum with investments in sensing technology for the brain and nervous system, Martha said.
“Sensing and closed loop technology is becoming foundational for the neuromod space,” said Martha, predicting Medtronic will grow market share and expand its lead in the $5 billion segment spanning treatments for chronic pain and movement disorders such as Parkinson’s disease, essential tremor, dystonia and epilepsy.
The company announced Monday that it received Europe’s CE mark for an adaptive deep brain stimulation (DBS) technology “that has been categorized as a brain-computer interface.” Martha said the device is the world’s first complete closed loop DBS system with self-adjusting brain stimulation for patients with Parkinson’s.
A submission to the Food and Drug Administration is in the works, he said.
Diabetes business turns around
Medtronic’s diabetes business has turned around and is growing in the double digits, according to Martha, boosted by its technology for patients on intensive insulin therapy. The company’s Simplera continuous glucose monitor sensor, which is half the size of its previous sensor and easier to use, is driving growth in international markets, he said.
Medtronic is pursuing expanded labeling for some products, including for patients with Type 2 diabetes, the CEO said.
Automated insulin delivery is becoming the preferred method to improve outcomes for patients with Type 1 diabetes, Martha added, and medical guidelines will “rapidly move the therapy to a standard of care.”