Dive Brief:
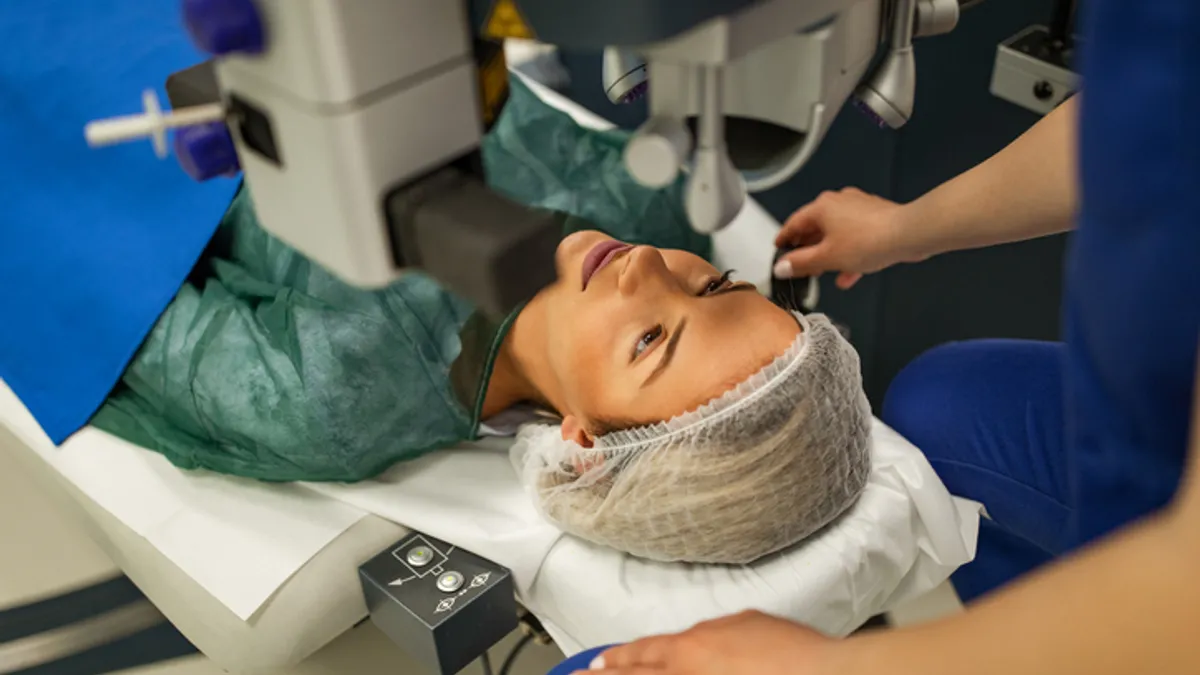
- The Food and Drug Administration’s draft guidance on the laser-assisted in situ keratomileusis, or LASIK, surgical procedures used to correct common vision problems has proven highly divisive.
- Medtech trade group AdvaMed took the “unusual step” of asking the FDA to withdraw the draft because of “numerous concerns” with the text. The American Society of Cataract and Refractive Surgery also called for the withdrawal of guidance it branded “biased and misleading.”
- Other organizations including the Society for Excellence in Eyecare and the Office of the Attorney General of Kentucky spoke out against the draft. Still, a few comments were more positive. The American Optometric Association, or AOA, called the proposal “timely and beneficial.”
Dive Insight:
The FDA published draft guidance on LASIK earlier this year in response to concerns that patients are not receiving information on the procedure’s benefits and risks. In the draft, the agency proposed a format and content of patient labeling that covers the suitability of an individual for LASIK and the risks associated with the procedure.
In the days and weeks after publishing the draft, the FDA received a torrent of feedback. Many of the 635 comments are from individuals who have undergone the procedure. The experiences described in the patient feedback run the gamut from LASIK was “an amazing experience that had long lasting positive effects,” to LASIK “destroyed my life.”
Individual physicians also commented on the draft. John Spaeth, an optometrist who has recommended patients for LASIK for two decades, called the draft “a grave mistake,” adding the information in the text “is misinformed, some is incorrect, and is often misleading.” Having sent three or four people a month for LASIK, Spaeth wrote that his practice has yet to see an adverse event or an unhappy patient.
There are several comments from medtech trade groups and healthcare professional societies, most of which are against the draft. AdvaMed’s opposition to the proposal reflects a range of concerns, including a claim there is “no clear rationale for attempting to so closely control the scope and nature of information provided to patients, or to dictate and verify communications between doctor and patient.” Kentucky’s attorney general’s office also criticized the FDA for regulating “a doctor’s practice of medicine.”
Amid the dissenting voices, the AOA’s response stands out. The body, which represents 48,000 doctors of optometry, optometric professionals and optometry students, whose members prescribe and often sell eyeglasses and contact lenses, called for some changes and clarifications but expressed broad support for the draft.