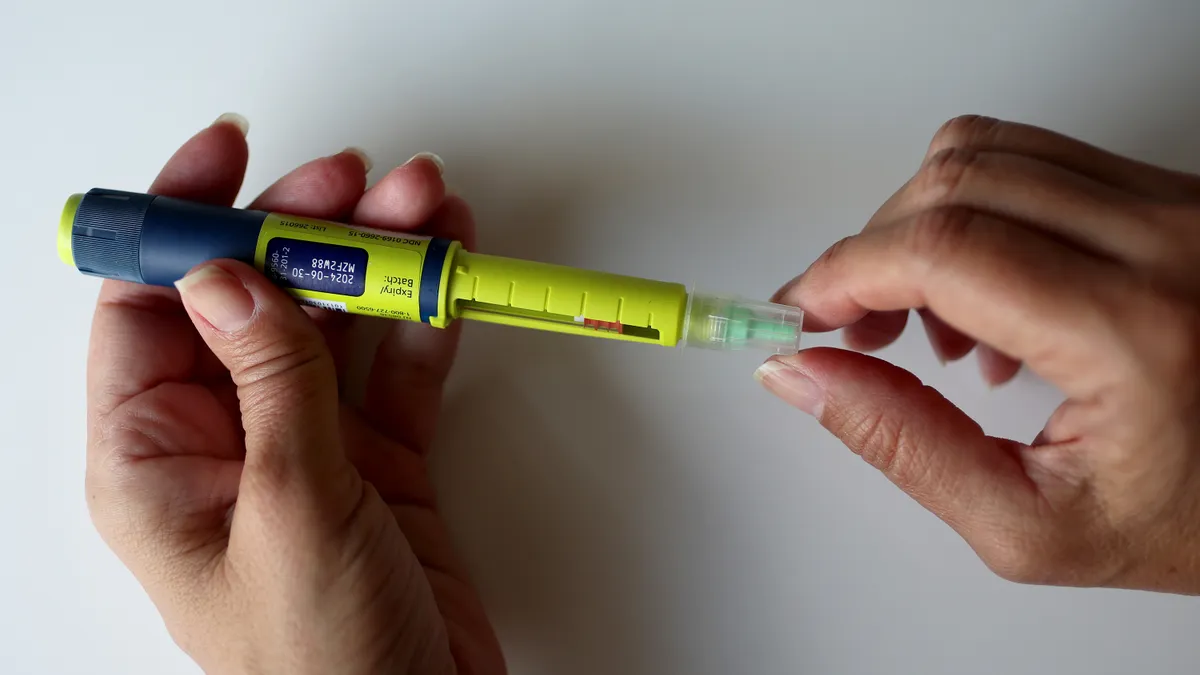
Arianna Gehan often starts her day by waking up to alerts from the devices she uses to manage her diabetes.
“A few days ago, I received over 60 notifications on my phone from my insulin pump and continuous glucose monitor,” Gehan said at a session convened by regulators gathering input on how to improve home healthcare for diabetes patients.
“Alarm fatigue is real, and diabetes burnout is a serious issue,” said Gehan, the co-founder and CEO of Daia Diabetes, developer of an app to share critical blood sugar information to emergency contacts.
Last Thursday, Food and Drug Administration officials listened to stories from patients like Gehan and medical device companies to kick off the agency’s Home as a Health Care Hub initiative.
Michelle Tarver, the new acting director of the FDA’s Center for Devices and Radiological Health, launched the initiative in April to advance health equity, starting with a focus on diabetes. The agency will partner with architecture firm HKS to make virtual prototypes of different living spaces — a single family home, an apartment and an accessory dwelling unit — to help medtech developers consider people’s living conditions as part of device design.
“Intentional development that includes members from different geographies, different resources, different cultures, different generations, different genders and different lived experiences helps us to ensure that the solutions developed can meet the needs of all,” Tarver said during the event.
Former CDRH Director Jeff Shuren, who announced his retirement last week, said the FDA started with diabetes because it affects more than 38 million people in the U.S., and that number is growing. The initiative stems from agency efforts to improve access to care and facilitate diversity in clinical trials.
“A model of healthcare that is focused on the system and healthcare providers squarely puts the burden of care and the coordination of care, the screening for complications and the prevention of poor outcomes more and more on the backs of the person who's already managing a complicated condition,” Shuren said.
A day in the life
Gehan shared her day-to-day experiences living with Type 1 diabetes during the meeting.
At home, Gehan often keeps three months of supplies, plus emergency supplies. She bought a backup generator for her house to ensure her insulin is refrigerated, even in the event of a power outage. Gehan explained that insulin could go bad if not kept at the correct temperature, creating a life-threatening situation.
Gehan also told regulators access to virtual care is an important innovation for diabetes management. She was able to do televisits with an endocrinologist at the height of the COVID-19 pandemic, but access has diminished, especially across state lines.
Courtney Lias, acting director of CDRH’s office of in-vitro diagnostic devices, shared how feedback from the diabetes community has helped regulators’ past decisions. For example, the FDA encouraged manufacturers to develop wireless data transmission, eliminating the need for “a mess of tangled cords” for users to manually download their glucose data. It also encouraged integration with mobile apps, removing the need to carry a separate receiver to get CGM readings.
“Learning these things made us realize that there were simple things to do in the device regulatory arena through working with patients, doctors and device developers to improve the lives of these patients by easy technology fixes,” Lias said.
Feedback from the diabetes community also was critical to the FDA approving the first automated insulin dosing systems in 2016, which allow CGMs and pumps to communicate and deliver insulin as needed.
Cost, access concerns remain
Other stakeholders at the meeting supported the initiative, but also flagged challenges with the safety and cost of home devices.
“For this initiative to impact public health in the way it's attended, cost definitely needs to be considered right from the start,” said Julianne Lally, associate director of community screening and clinical trial education for diabetes nonprofit Breakthrough T1D, formerly known as JDRF.
Lally cautioned that it may take longer than expected for new devices to be adopted, even if their benefits are “widely known and shared,” citing the example of CGMs.
The devices are recommended by the American Diabetes Association once patients start insulin treatment, but studies have estimated that less than half of people living with Type 1 diabetes use a CGM, Lally said, and there are significant socio-demographic disparities.
René Quashie, vice president of digital health at the Consumer Technology Association, reiterated Lally’s concerns about costs, focusing on the role of insurance and concerns about lack of reimbursement.
“I think we need to be thoughtful about this because we may end up in a situation where a lot of these incredible tools, especially as they get more sophisticated, are going to end up in the hands only of those who can afford the out-of-pocket costs,” Quashie said.
Scott Lucas, vice president for device safety at nonprofit ECRI, said complex medical devices are often designed for healthcare professionals rather than home users. Device companies need to think about user interface, as well as power supply, internet access, space, literacy and language barriers, he said.
Lucas also raised concerns with gaps in communication about recalls, noting device manufacturers seldom communicate directly with patients. For these reasons, ECRI listed usability challenges with home devices as a top technology hazard in 2024.