Dive Brief:
-
FDA has authorized Signifier Medical Technologies' eXciteOSA device for use in the reduction of snoring and mild sleep apnea.
-
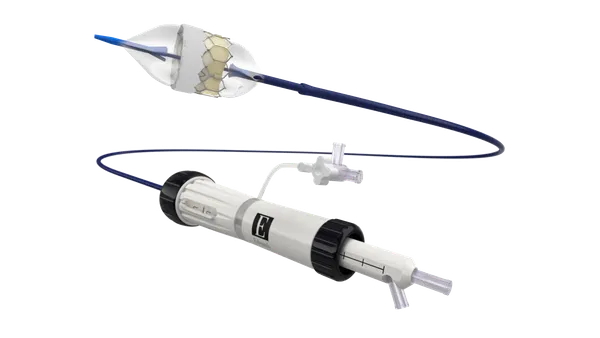
Unlike existing treatment options, patients use the product while awake. The device delivers neuromuscular stimulation to improve tongue muscle function and thereby prevent airway obstruction during sleep.
-
Signifier got the device authorized via the De Novo pathway after running a clinical trial of 115 patients. The study linked the device to reductions in loud snoring and scores on a scale of obstructive sleep apnea severity.
Dive Insight:
FDA has authorized more than 100 oral appliances sleep apnea patients can wear while sleeping to manage their conditions, according to a patient group. These devices, worn like mouthguards used by players of contact sports, hold the lower jaw forward during sleep to prevent the tongue from collapsing and blocking the airway.
Signifier is coming at the problem from a different angle. Rather than reposition the mouth to stop the tongue collapsing, Signifier wants to strengthen the muscle so it stays in place without night time support.
The eXciteOSA mouthpiece delivers electrical muscle stimulation via four electrodes, two above the tongue and two below the tongue. Users wear the mouthpiece for 20 minutes a day for six weeks and once a week thereafter. Inspire Medical Systems also sells a neurostimulation device but it is cleared for use in moderate-to-severe obstructive sleep apnea.
To evaluate the efficacy of the device, Signifier enrolled 115 patients with snoring in a clinical trial. Forty-eight of the patients had snoring and mild sleep apnea. After six weeks of treatment, 76% of the patients experienced a 20% or greater reduction in time spent snoring at 40dB or louder.
In the subset of sleep apnea patients, average scores on the Apnea-Hypopnea Index, a measure of the severity of the condition, fell by 48% in 41 out of 48 participants. The average scores fell to 5.27 on a scale that classes mild sleep apnea as any score between five and 15.
The device was well tolerated, with excessive salivation, tooth discomfort and tongue tingling among the most common adverse events. FDA's authorization prohibits the use of the device in particular patient populations, including individuals with pacemakers, dental braces and ulcers in and around the mouth.
Having secured De Novo authorization, Signifier now faces the challenge of establishing eXciteOSA in the congested snoring and sleep apnea market. The American Academy of Sleep Medicine supports the use of oral appliances in some sleep apnea patients and CPAP, the first-line option for many other individuals, has shown efficacy in people with mild forms of the condition.
Companies including ResMed and Philips compete for the CPAP market. ResMed and tens of other companies sell oral appliances. Signifier raised $10 million in September to challenge for the market.