Dive Brief:
- The U.S. Food and Drug Administration has granted 510(k) clearance to Huma Therapeutics’ Software as a Medical Device (SaMD) platform.
- According to Huma, the clearance permits it to host digital health tools that support screening, diagnosis, dosing recommendations and clinical decision making on its SaMD platform.
- Huma CEO Dan Vahdat said the clearance will enable teams, from two-person startups up to big pharma companies, to launch digital health interventions in days “with almost no cost.”
Dive Insight:
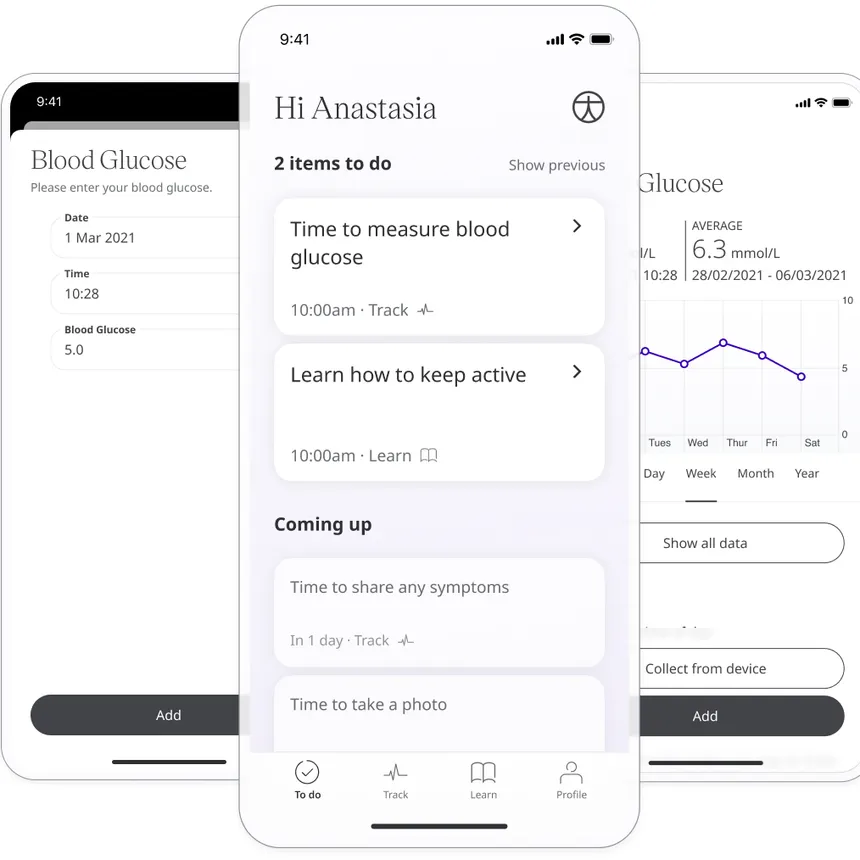
Huma is the developer of a “hospital at home” platform designed to collect real-world data remotely and connect patients and clinical teams virtually. The company raised $130 million in 2021 and landed a partnership with Bayer earlier this year.
“Currently, it's very expensive to innovate. Usually it takes two, three, four years for digital interventions, and can cost tens of millions of dollars. With our class II FDA clearance, ... two people in [a] garage ... can launch solutions, digital health interventions for a very new disease, in [a] matter of days, with almost no cost,” Vahdat said in a video discussing the news.
The platform can monitor patients and connect to devices such as smart inhalers.
The FDA cleared the device through the eSTAR program that it set up to improve the quality of medtech submissions. Use of eSTAR electronic submissions will be mandatory for 510(k) filings starting in October, unless a submission is exempted.
Huma also received 510(k) clearance for its cardiovascular risk score algorithm. The Bayer partnership covers a tool that uses an individual's answers to 15 questions to predict if they are at risk of developing cardiovascular disease over the next 10 years.












