Dive Brief:
- Johnson & Johnson said Friday it launched Velys Spine, a surgical robot and standalone navigation platform.
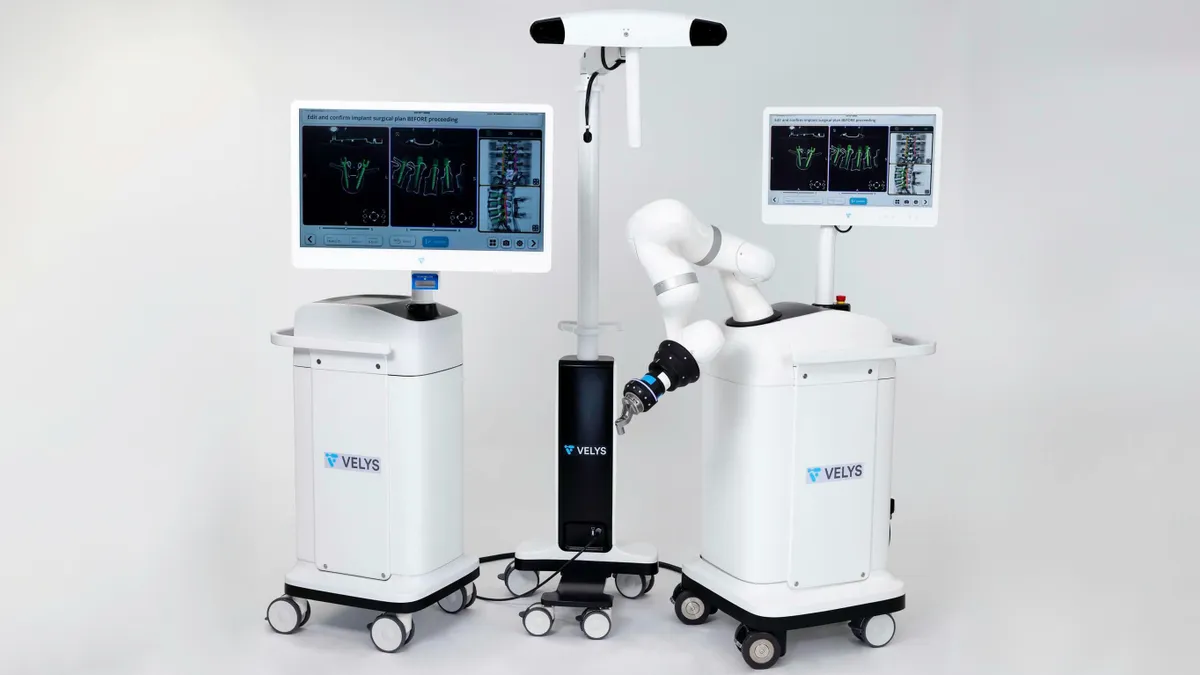
- The 510(k)-cleared system, which J&J developed with eCential Robotics, is designed to provide guidance on the placement of screws in freehand and robotic-assisted spine surgeries.
- J&J’s Depuy Synthes plans to make the system available commercially in the first half of 2025. The system will join other Velys offerings J&J has cited as a growth driver in its hip and knee businesses.
Dive Insight:
ECential, a French company specializing in surgical robotics, received 510(k) clearance from the Food and Drug Administration for a spine technology in June. The FDA clearance covered a spine navigation and robotic-assistance device that is compatible with specific Depuy Synthes instruments. ECential created the system to guide the instruments in one of two modes, freehand or robotic assisted guidance.
The device supports spinal fusion procedures in the cervical, thoracolumbar and sacroiliac spine. Users can plan surgeries using three-dimensional images and then receive robotic guidance on the placement of spinal bone screws during procedures. Surgeons can use Depuy Synthes’ instruments from product lines including Trialtis, Symphony and Viper Prime, J&J said in the announcement.
Tim Schmid, J&J’s worldwide chairman of medtech, teased the launch on the company’s earnings call in July, telling investors that “in short order, you will see news about our commitment to really bringing robotics to other parts of the orthopedics portfolio, especially in spine.”
Schmid made the comment while discussing what he called “the resurgence of orthopedics.” Asked by an analyst to explain why hip and knee growth has improved, Schmid said enabling technologies such as Velys Hip Navigation and the automated total hip arthroplasty device Kincise are drivers of the rebound.
The company’s hip sales grew roughly 5% year over year to $417 million in the second quarter, and knee sales increased by 8.4% to $394 million.
J&J won 510(k) clearance for its Velys surgical robot in partial knee replacement procedures in June. The robot is part of J&J’s attempt to take market share from Stryker and Zimmer Biomet, including by focusing on ambulatory surgery centers.