Dive Brief:
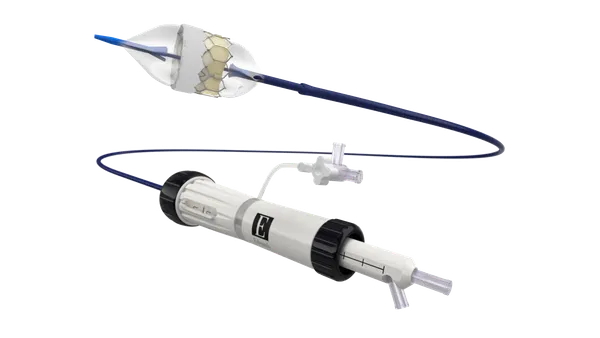
- Medtronic said Monday it received Food and Drug Administration approval for deep brain stimulation (DBS) technology that is implanted while the patient is under general anesthetic.
- Surgeons traditionally perform DBS procedures, a treatment for medical conditions like epilepsy and Parkinson’s disease, while the patient is awake. However, a series of studies have shown there may be benefits to putting the patient to sleep for the procedure.
- Medtronic said it is the first company to receive FDA approval to offer DBS surgery while a patient is asleep or awake. U.S. surgeons have used DBS devices off-label in asleep patients for years.
Dive Insight:
Medtronic’s brain modulation business reported low double-digit growth year over year for the quarter ending April 26, its most recent earnings report. CEO Geoff Martha said in a May earnings call that the company saw “strong uptake” of Percept RC with Brainsense technology, a rechargeable device that captures brain signals and delivers stimulation.
Amaza Reitmeier, Medtronic’s general manager of brain modulation, said in a statement that the asleep approval is a “significant advancement in our surgical offering.” The authorization provides a potential point of difference over rival devices from companies such as Abbott and positions Medtronic to target an emerging opportunity in the DBS market.
Interest in asleep DBS has increased in recent years as surgeons have generated evidence of its benefits in certain situations. In 2021, the Journal of Neurosurgery published data on 103 patients a single surgeon treated with asleep DBS at Brigham and Women’s Hospital from 2015 to 2019.
The authors said there are “distinct advantages” to asleep DBS, such as shorter procedures, but also noted situations in which the traditional approach is preferable. Asleep DBS requires specialized equipment and use of an intraoperative scanner, the authors said, and does not allow intraoperative stimulation to confirm appropriate target placement.
Clearpoint Neuro provided the neuronavigation system used at Brigham and Women’s. Answering an analyst question in 2021, Clearpoint CEO Joe Burnett said “some of the materials from different DBS companies are starting to indicate more and more usage of really leaving it up to the physician as to whether the patient should be awake or asleep for these procedures.”