Dive Brief:
- Medtronic has become the first company to receive Food and Drug Administration approval for a drug-eluting stent in bifurcation percutaneous coronary intervention.
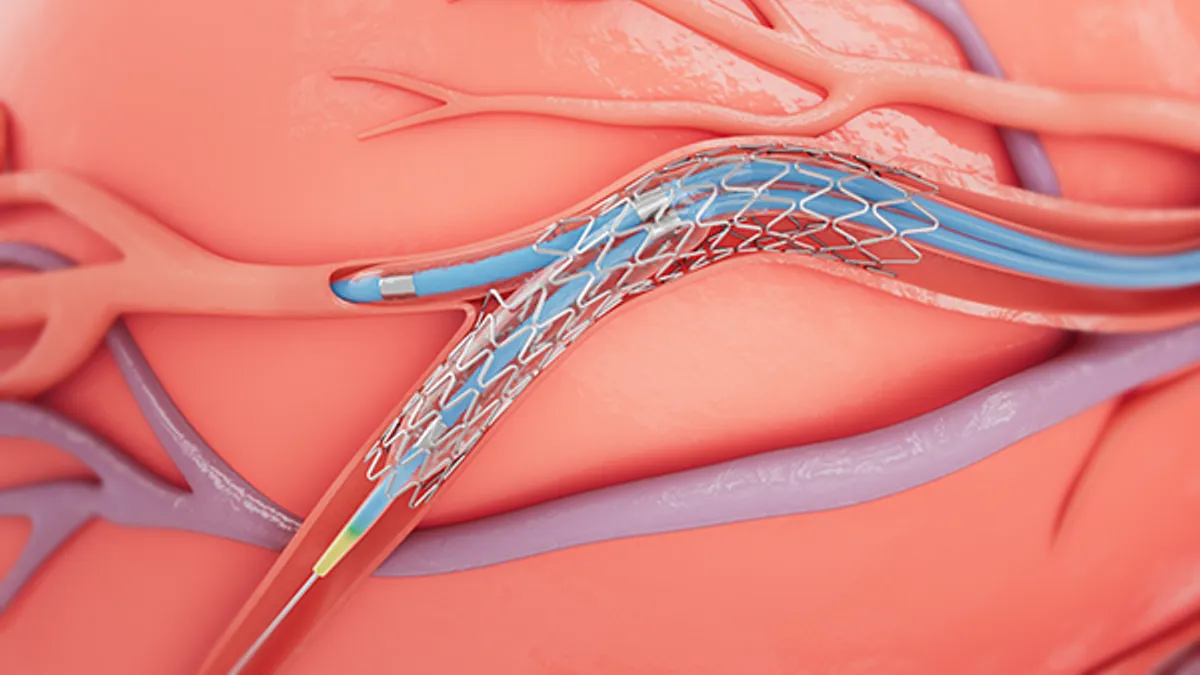
- The approval covers the use of a single Onyx Frontier and Resolute Onyx stent to treat non-left main bifurcation lesions, which occur when plaque accumulates around a division of a major coronary artery.
- Researchers have found the one-device, provisional stenting method delivers better results than two stents in some patients, while physicians lacked a device specifically indicated by the FDA for the application.
Dive Insight:
Medtronic demonstrated the suitability of its Onyx Frontier and Resolute Onyx stents for provisional stenting of bifurcation lesions in a clinical trial that enrolled 205 patients. The report said the target vessel failure rate one year after treatment, at 6.9% was “significantly lower than the pre-specified performance goal.” The one-year rates of cardiac death and heart attack involving the target vessel were 1.5% and 2.9% respectively.
Having presented the data last year, Medtronic now has secured approval for the use of its stents in the treatment of non-left main bifurcation lesions. The decision gives physicians access to stents that are FDA approved for use in lesions that are typically associated with lower success rates and increased rates of long-term adverse cardiac events.
According to Medtronic, the single-wire design of its stents makes them well-suited to the treatment of bifurcation lesions. Because a single strand of wire is formed into a wave to construct the stent, the company argues its devices make it easier to access side branches and are otherwise a good fit for complex bifurcation anatomy.
The stents are part of a portfolio of coronary and peripheral vascular devices that dragged on Medtronic’s financial performance in its most recent financial results, when the business unit posted a 7% decline in reported sales. Medtronic attributed the performance to the continuing impact of COVID-19 on percutaneous coronary intervention market procedures in several regions around the world.