Dive Brief:
- Procept Biorobotics has received 510(k) clearance for an updated version of its surgical robot for prostate procedures, the company said Wednesday.
- Like its predecessor, the device delivers aquablation therapy to treat noncancerous prostate enlargement. Procept has added artificial intelligence features, switched to a single-use scope and made other changes intended to support mass-market adoption of the device.
- Wall Street analysts were surprised by the timing of the clearance, with the team at Truist Securities saying a 2025 or 2026 launch was expected, but largely welcomed the news as a potential growth driver. Procept’s share price closed up 28% at $83.44 on Wednesday.
Dive Insight:
Procept received de novo authorization for its first-generation Aquabeam device in 2017. The surgical robot delivers a pressurized jet of fluid to cut and remove prostate tissue in men with lower urinary tract symptoms caused by benign prostatic hyperplasia. Around 400 hospitals are using the device, Chief Commercial Officer Sham Shiblaq said Wednesday on a call with analysts to discuss the 510(k) clearance.
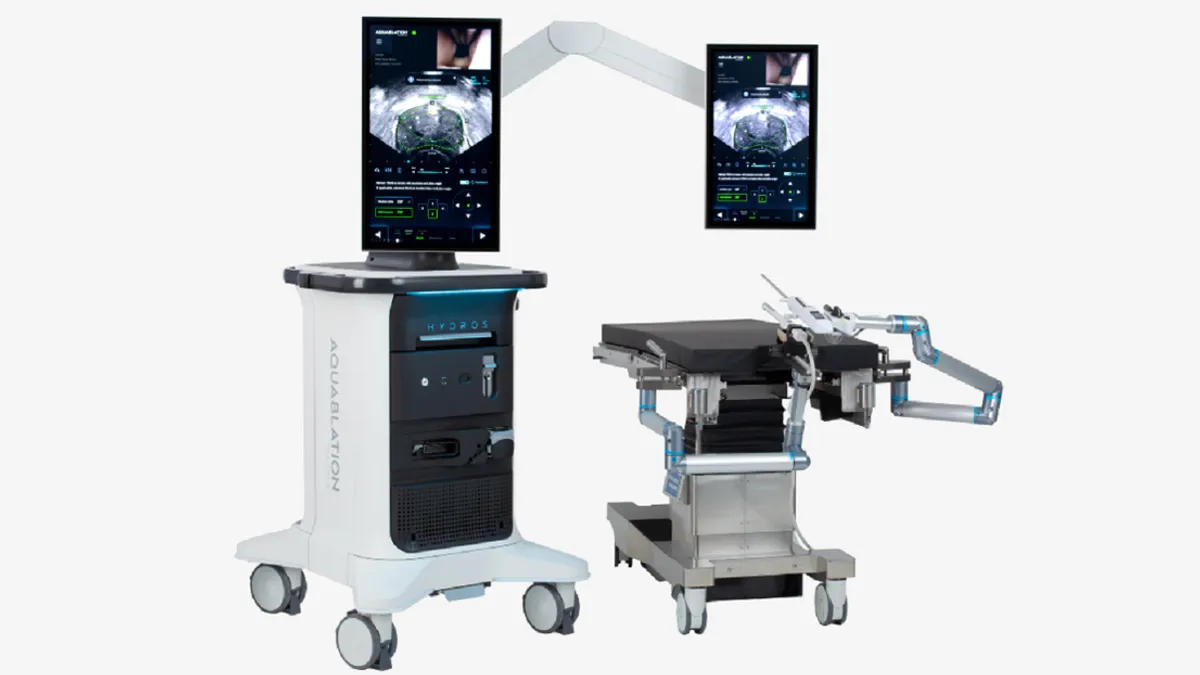
The next-generation device, Hydros, is designed to build on the experiences of early adopters who have performed 50,000 procedures using the old system. By applying the lessons to Hydros, Procept has tried to create a product that is suitable for use by a larger number of surgeons and healthcare facilities.
Shiblaq highlighted three new features. Hydros incorporates AI that acts as a “virtual ultrasound expert that's side-by-side with the surgeon,” Shiblaq said, and helps the physician to interpret ultrasound images for key anatomical landmarks. The AI improved the accuracy of landmark identification of everyone from novice users to experienced aquablation surgeons in Procept’s testing, Shiblaq said.
The second key feature is the integration of the system with “an advanced ultrasound system in a new digital cystoscope,” the Procept executive said. The update is designed to improve visualization and make setup more streamlined. Finally, Procept is eliminating the need for scope reprocessing by providing a preassembled handpiece with a single-use digital scope.
The updates are intended to make the technology simpler to adopt and “make it easier for room turnover,” Shiblaq said. Procept will initially target new customers before starting to switch existing users to the new system in 2025. Beginning in the fourth quarter, the company will only sell the new device.
Leerink Partners analysts said in a note to investors that they expect the AI feature to “be a key driver of incremental adoption as the system is rolled out broadly.”
William Blair analysts also published a positive note, in which they identified Hydros as “another tailwind to near- and medium-term growth since it lowers the barrier to adoption.” However, BTIG analysts called out factors that could constrain uptake.
“We have reservations on initial demand into 2025 as [management] has yet to identify specific incremental clinical benefits with Hydros coupled with the continued market segmentation by prostate size which may not necessitate such a capital outlay,” the analysts said in a note to investors.
Barry Templin, who leads technology and clinical development at Procept, said on the call that the company has not conducted clinical studies of the new system. More reproducible interpretation of ultrasound could provide clinical benefits, Templin said, and the company will monitor that as it rolls the new product out.