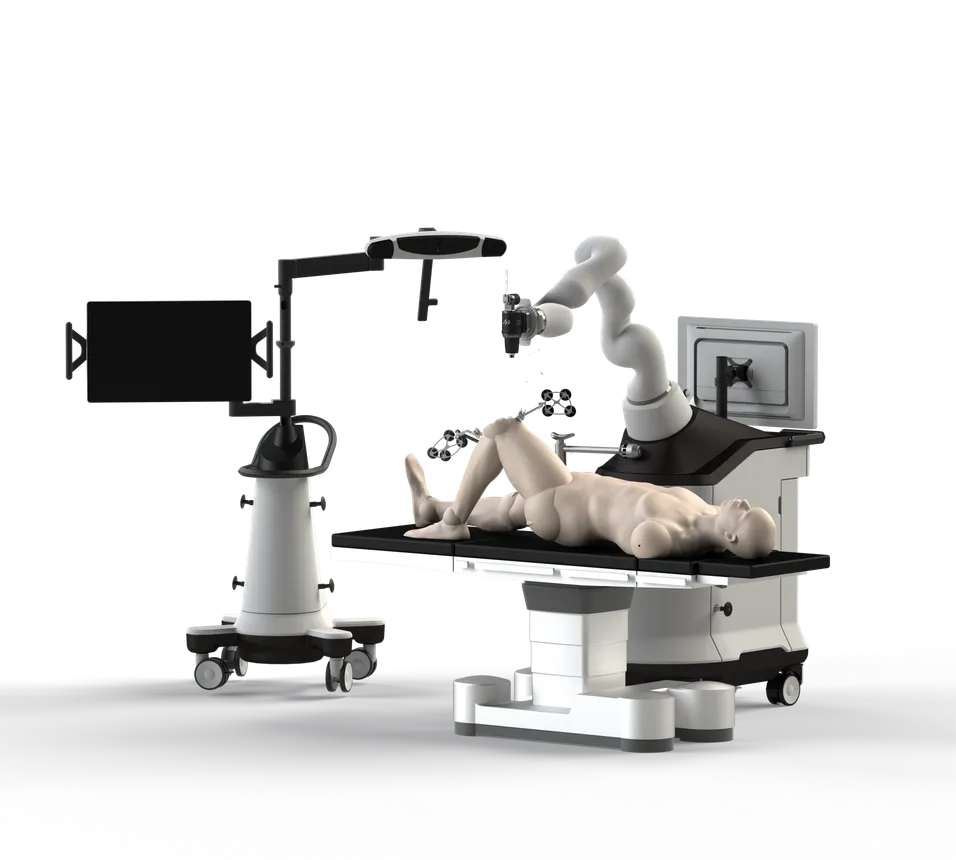
Seven-year-old surgical robotics company Monogram Orthopedics, which went public in May, is aiming to take on medtech heavyweights such as Stryker and Zimmer Biomet as well as smaller challengers in the growing market for robot-assisted joint replacement. CEO Benjamin Sexson, an engineer by training, sees a pressing need for personalized implants made to fit each patient’s specific anatomy. He explained to MedTech Dive how the robotic platform that Monogram is developing could help reduce complications associated with knee replacement surgery, and how the Austin, Texas-based company plans to compete against Stryker’s market-leading Mako robot.
This interview has been edited for length and clarity.
MEDTECH DIVE: What are some of the challenges in knee replacement that you are trying to solve with your surgical robot?
BENJAMIN SEXSON: The idea is to move away from what's called mechanical alignment, which is how we've always done it with manual instruments. A lot of the robots on the market today are just basically helping surgeons put the knee into mechanical alignment – everybody gets the same knee, the joint line is basically restored to be parallel to the floor.
With functional, or more personalized, alignment approaches, surgeons are tweaking the plan based on, I have my patient’s specific data, I have the CT scan, and I can assess intraoperatively what do I think looks best? And they can make very, very precise and accurate tweaks to the surgical plan that, over time, we're seeing are enhancing patient satisfaction.
Stryker and Zimmer both have the ability to use a CT scan or advanced imaging to preoperatively plan. Stryker has been driving this trend. Stryker is showing that patients are happier when you can do some degree of personalization of the surgery.
Other players in the space have been struggling [because] they are imageless. They made the bet that insurance companies would not be as accommodative in the future to reimbursing the cost of the CT scan, and so their surgeries correlate to generalized models. We think that over time, this is going to be increasingly problematic for those other players.
How will you compete against Stryker?
In our assessment, the most differentiating feature of the Mako system is safe, reliable and efficient robot-enabled cutting. They have a robot-mounted sagittal saw that a surgeon is moving around within a constrained plane. And it's just very efficient at removing bone. But there are some serious limitations to their arm that we see.
The first limitation is that their system only has four joints, which is fine for doing a knee. But it's not fine for many other applications where you need more dexterity out of the arm. So if you look at robotic utilization in hips, only 3% of hips today utilize advanced technology. Stryker has not had meaningful penetration in the hip market.
To actually do a hip surgery, you need a minimum of six joints for the robot. When you start to think about other applications, like shoulders and extremities, it really becomes a bit limiting. So, the Stryker Mako robot is a knee robot. But we think it's much less efficient for other orthopedic applications.
And we think it's really problematic to have all of these kinds of application-specific robots. We think what's much more efficient is to have a single robot that can do any type of orthopedic surgery. So that's one distinct advantage of our system.
The second distinct advantage is that our system is active versus haptic. The surgeon doesn't have to move the arm around, the robot is executing a very, very safe cut path, and the surgeon is driving the robot along this cut path with a foot pedal. They have both hands unencumbered.
We will have to match the efficiency and safety of [Stryker’s] cutting and optimize the workflow to be more efficient. Fast and efficient cutting is a substantial focus for Monogram, and we have broken new ground by actively cutting with a sagittal saw. So our vision is really to make orthopedic surgery completely idiot-proof, where it doesn't matter if you're a resident doing a surgery for the first time or a very seasoned orthopedic surgeon. It's an autonomous system.
What we're working on for our next generation that we think will be really the killer app is to go what's called marker-less. We're developing a technology where the camera will be able to directly track the bone and will not have any markers. And the advantage of this is, it will allow us to do surgery very, very quickly. We envision a future where you make an incision, the camera can very quickly tell where the bone is without needing to install any other equipment, and you can start cutting.
What gives you confidence that there is room for another robot in the orthopedic marketplace?
Investors generally [say], “Well, everybody already has an orthopedic robot.” OK, but really only two companies are CT-based, and only one of those companies is actually [using surgeon-initiated] cutting. So there's really only one navigated, efficient, cutting, CT-based robot, and that’s Stryker, and they have 90% of the market. And only 10% of knees today are robotic. So there's a huge opportunity.
It's inefficient for a small-footprint private clinic to be forced to buy five-plus different robots, one that's good for the spine, one for the hips, one for the knees, one for the shoulders, one for the ankles, etc. The fundamental difference between our philosophy and our competitors is that Monogram seeks to develop one robot optimized for many applications.
Why did you decide to focus first on the knee market?
I'll start by saying that our robot is not a knee robot. It's an orthopedic robot. It just so happens that knees are our first application for a number of reasons. One is that we didn't want to go on a missionary effort trying to convince people in, say, the shoulder space, to use an orthopedic robot and develop a whole new surgery. We wanted to come into a market that already had billing codes and an established market presence and some level of acceptance.
There are about a million knee replacements a year in the United States, and about 10% of those fail every year. And a lot of people are not satisfied with their knee replacement. We think we can make it a lot less scary and a lot less risky.
Where are you in the process toward gaining FDA approval?
We've had what's called a pre-submission meeting with the FDA. This is a 510(k) submission, so we're going to be claiming substantial equivalence to a system that's already on the market. Where we are is, the FDA is accepting of our clinical pathway. They're accepting of our predicate [Stryker’s robot] and intended use. The question that's still outstanding is whether or not the technical differences with our predicate need to be tested with clinical data. Our plan is to take a defensive approach and submit to the FDA with clinical data. Our plan is to lean heavily on capturing that data outside the U.S.