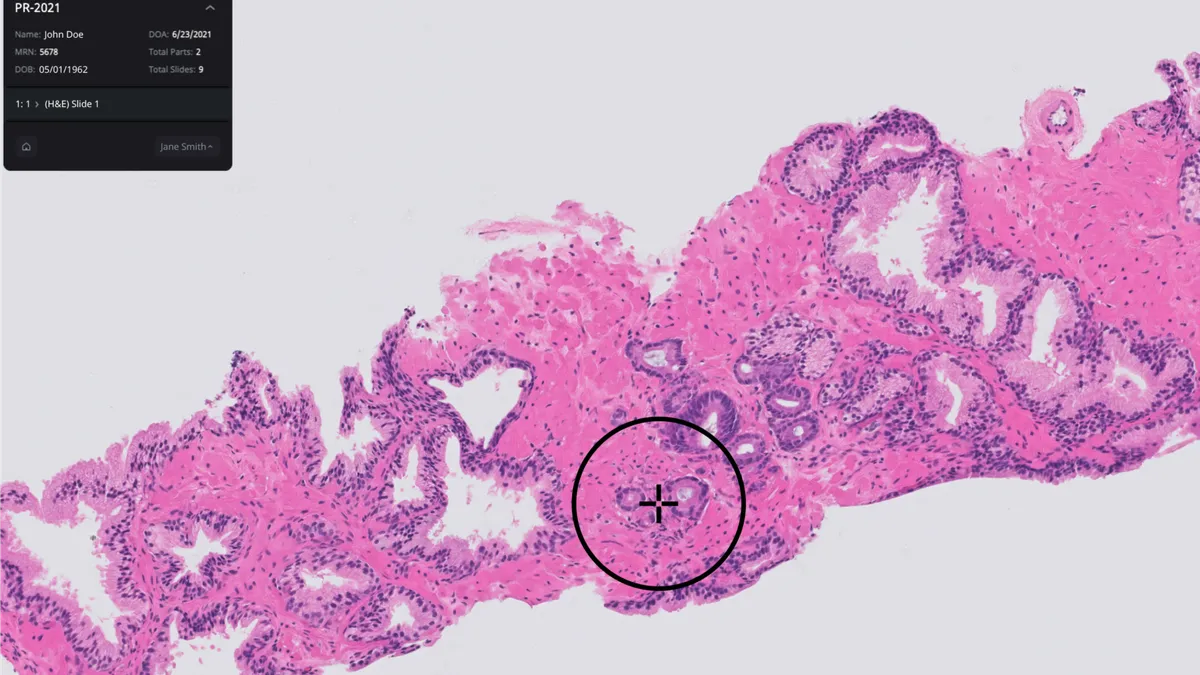
Roche's Foundation Medicine leads the latest group of FDA breakthrough designations, securing the regulatory privileges for an assay designed to detect circulating tumor DNA (ctDNA) in plasma.
FDA has awarded breakthrough status for the test in the detection of molecular residual disease (MRD) in early-stage cancer after curative therapy. With some therapies eliminating tumors, but leaving residual disease, a patient's MRD status can inform whether further treatment is needed and help assess the risk of relapse.
Foundation Medicine developed the assay, FoundationOne Tracker, with Natera. The companies teamed up in 2019 to use components of Natera's Signatera platform to develop monitoring assays for use by biopharmaceutical and clinical customers who order the pan-tumor liquid biopsy FoundationOne CDx, and then went on to make the test available for research use last year.
FoundationOne Tracker is one of two liquid biopsies in the latest batch of FDA breakthrough designations. The agency also granted the regulatory status to a Datar Cancer Genetics blood test for detecting early-stage prostate cancer. Datar Cancer Genetics previously received FDA breakthrough status for its early-stage breast cancer test.
The FDA's latest group of breakthrough designations also features two treatments for epilepsy. NeuroSigma won breakthrough status for its Monarch eTNS System for adjunctive use for reducing the frequency of seizures in people 18 years of age or older. It's intended to treat partial-onset seizures that don't respond to two or more antiepileptic medications.
NeuroSigma's Monarch eTNS provides external trigeminal nerve stimulation. The device, which is already cleared for use in the treatment of pediatric ADHD, reduced the frequency of seizures in patients with drug-resistant epilepsy in a small clinical trial.
Precisis picked up the other epilepsy-related breakthrough device nod from FDA, securing the status for its EASEE brain stimulator. The device consists of electrodes that are positioned on the skull bone under the scalp. By delivering current to a specific area of the brain, EASEE is designed to inhibit epileptic seizures. Precisis is pitching the device as a simpler, less invasive alternative to stimulation of the vagus nerve and deep brain.
FDA also recently awarded breakthrough designations to cardiovascular disease devices. Rapid Medical secured the regulatory privileges for its Comaneci embolization assist device in the treatment of cerebral vasospasm following hemorrhagic stroke. Vasospasm describes the narrowing of the arteries. Comaneci is designed to enable physicians to temporarily expand blood vessels.
Cardiosense received the other recent cardiovascular disease breakthrough nod for an algorithm to spot patients at risk of decompensated heart failure. The algorithm analyzes data collected by the CardioTag device to noninvasively assess pulmonary capillary wedge pressure (PCWP). Cardiosense's breakthrough status comes months after VoluMetrix secured the same privileges for its noninvasive approach to PCWP, a measure that indicates if a patient has heart failure that currently requires invasive catheterization.
Theradaptive received breakthrough status for its OsteoAdapt SP Spinal Fusion implant in posterolateral spinal fusion to treat degenerative disc disease, spondylolisthesis or retrolisthesis. The implant is part of Theradaptive's bid to improve on the safety of existing devices for spinal fusion, orthopaedic repair or craniomaxillofacial repair, while reducing revision procedures, through the use of regenerative implants.
FDA granted breakthrough status to Bonalive Biomaterials' orthopaedic granules. The granules, which are made of a bioactive glass, are intended for use as a bone graft substitute that potentially protects against microbial colonization.
The agency also awarded breakthrough designation to Noninvasix's LIVOx Central Venous Oxygenation Monitor for use in adults at risk of septic shock. LIVOx is a noninvasive device that monitors central venous oxygen saturation, an indicator of septic shock, to try to facilitate the early treatment of the life-threatening condition.
Finally, DynamiCare Health received breakthrough device designation for a digital therapeutic designed to treat tobacco use disorder complicating pregnancy and childbirth. The digital treatment is based on a motivational incentives approach in which patients earn financial incentives for abstaining from smoking. As well as a smartphone app, the device consists of a connected breath carbon monoxide monitor, saliva nicotine tests and a reloadable debit card.