Dive Brief:
- Scopio Labs has received de novo authorization for a device that finds and presents images of cells in bone marrow smears.
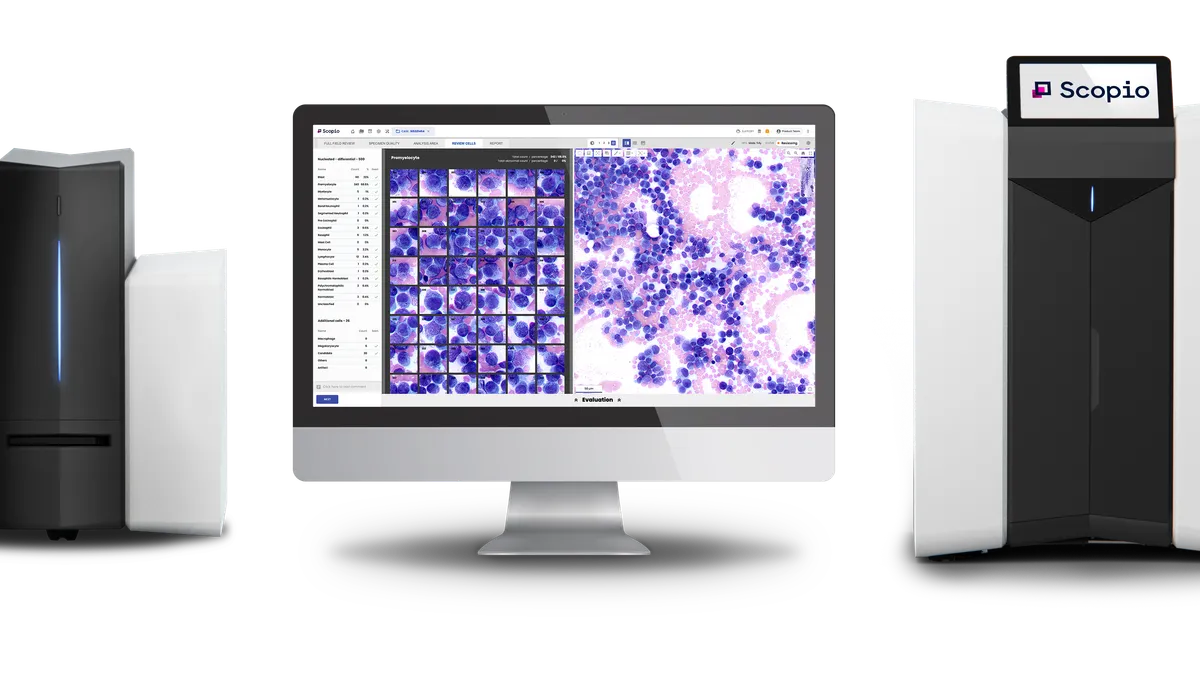
- The Food and Drug Administration authorization clears Scopio to add capabilities to its X100 and X100HT devices, imaging platforms that allow users to view blood samples digitally rather than under a microscope. Scopio announced the de novo clearance on Wednesday.
- Siemens Healthineers struck a deal to distribute Scopio’s platforms in 2023. The company identified the technology as a way to make laboratory workflows more efficient.
Dive Insight:
Scopio launched in 2015 with the goal of “developing the next generation of microscopes.” The company has passed a series of milestones over the past four years, receiving CE marks and FDA clearances, raising $66 million across series B and series C financing rounds, and striking deals with Beckman Coulter and Siemens.
The FDA granted 510(k) clearance to Scopio’s X100 device in 2020 and cleared its high-throughput sibling, X100HT, via the same pathway in 2022. The clearances covered the use of the devices for locating and displaying images of white cells, red cells and platelets acquired from fixed and stained peripheral blood smears.
The de novo authorization covers the application of X100 and X100HT to bone marrow aspirate. In that use, the devices automatically locate and present images of hematopoietic cells on a bone marrow aspirate smear obtained from patients being evaluated for blood diseases. Presenting the images could help trained operators identify and classify each cell according to type.
Scopio cited Medica’s Easycell Cell Locator as the predicate device when it sought 510(k) clearance for X100, but the bone marrow application breaks new ground, forcing the company to use the de novo pathway. Scopio’s bone marrow application combines high-resolution images with its AI-powered decision support software.
CEO Itai Hayut said in a statement that the technology can help labs to “streamline workflows, reduce operational costs and enhance patient care.” The company added that the software allows specialists to access and review smears remotely and helps facilitate second opinions.
The FDA created special controls as part of the authorization, such as performance data documentation and labeling requirements, enabling other companies to bring similar devices to market via the 510(k) pathway. The controls are intended to mitigate the risks of false positive and false negative results.