Dive Brief:
- Synchron’s brain computer interface (BCI) device caused no deaths or permanent increases in disability in the first year of a U.S. clinical trial, the company said Monday.
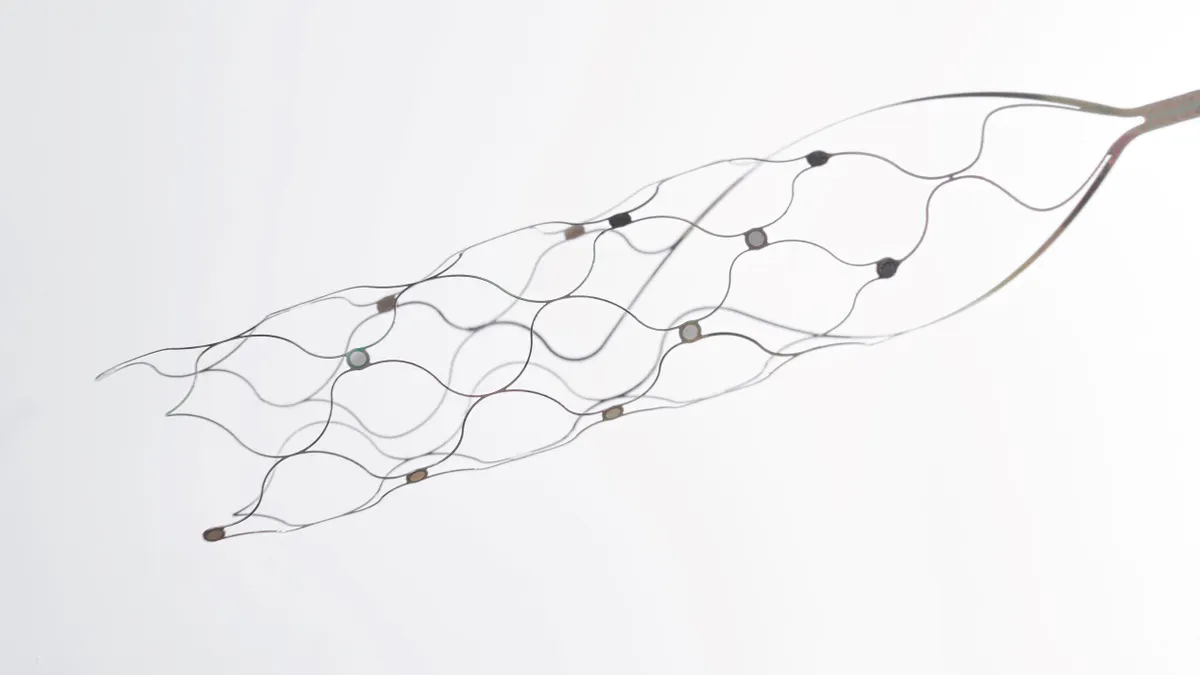
- The study enrolled six people with severe chronic bilateral upper-limb paralysis unresponsive to therapy to receive the device. The absence of device-related death or disability caused the trial to meet its primary endpoint.
- Synchron also reported evidence the technology consistently worked in participants, with the conversion of brain signals to motor outputs allowing people to perform digital tasks. The median deployment for the device was 20 minutes.
Dive Insight:
The Command early feasibility study is the first assessment of the device in the U.S. Synchron originally tested the BCI system in five people in Australia. After finishing the Australian trial in 2022, the company began enrolling people at two sites in New York and one location in Pennsylvania. Synchron designed the U.S. study to assess the safety of the device.
The co-principal investigator shared 12-month data at the 2024 Congress of Neurological Surgeons. In the trial, no device-related serious adverse events resulted in death or permanent increased disability during the one-year post implant evaluation period. No patients included in the analysis in the Australian trial experienced those outcomes either.
No patients in the U.S. trial had serious adverse events related to the brain or vasculature. Physicians accurately deployed the device and achieved target motor cortex coverage in the brain in all six patients.
While the study primarily assessed the safety of the device, Synchron also collected early evidence that its BCI system works as intended. The company said brain signals related to motor intent were captured consistently and transformed into digital motor outputs. That transformation allowed patients to carry out “a range of digital tasks,” Synchron said.
The company recently shared a video of a person with amyotrophic lateral sclerosis controlling their smart home via a connection between his BCI and Amazon Alexa. The same patient has used the implant to control the cursor on the Apple Vision Pro.
Synchron launched a patient registry in April to connect with patients as it prepared for the next stage of clinical trials. The update about the Command trial makes no mention of further studies, but CEO Tom Oxley recently told Reuters that Synchron plans to enroll dozens of participants in its next trial.