ST. PAUL, Minn. — Mizuho brings the Cerevention™ MindsEye™ Expandable Port to the U.S. market
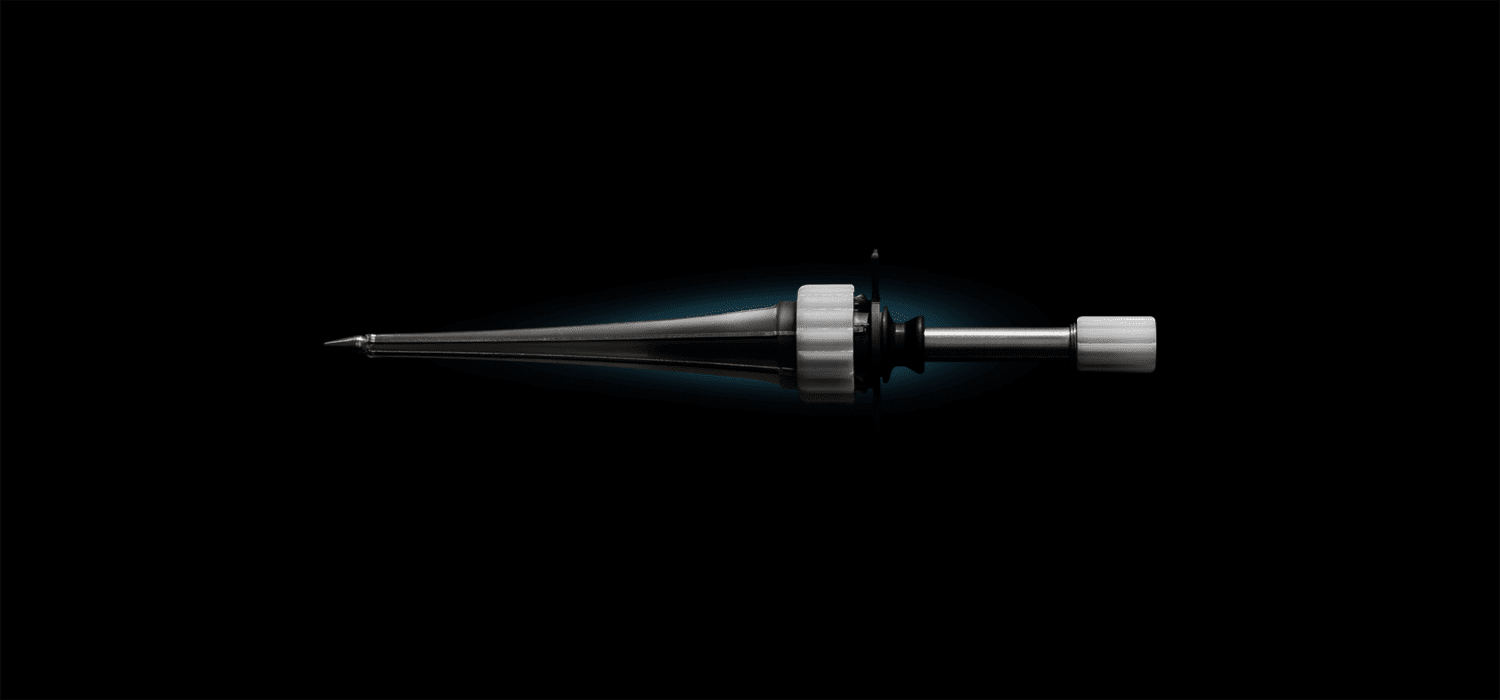
SAINT PAUL, Minn. (Aug. 17, 2022) – The Cerevention™ MindsEye™ Expandable Port, the world’s first minimally invasive, expandable port providing deep brain access and visualization during surgical treatment of stroke, cancer, and other conditions, is now on the market, after successful human use.
Mizuho America has secured an exclusive agreement to distribute the MindsEye expandable port to neurosurgeons in the U.S.
“Mizuho’s mission is to deliver the best devices to neurosurgeons,” said William Delaney, vice president of sales at Mizuho America. “The MindsEye expandable port is a device like no other. We look forward to bringing it to the market by leveraging the strength of our neuro portfolio and distribution channel to make an immediate impact on the ease of procedures for surgeons and quality of life for patients.”
The device is being used in human treatments at Tulane University by Dr. Art Wang and Dr. Johnny Delashaw, who conducted rigorous comparisons of options for minimally invasive neurosurgical procedures before choosing the MindsEye expandable port. “Its benefits to neurosurgeons, such as expandability, easier insertion and removal, and transparency that minimizes glare and allows surgeons to see surrounding tissue, are truly unique. The MindsEye expandable port has raised the bar for standard of care in neurosurgery and will improve patient outcomes.”
“This is next-generation deep brain access technology,” said Dr. Mario Zuccarello, professor of neurosurgery at University of Cincinnati Medical Center, who collaborated on the development of the device. “It’s distal flare design supports real-time changes in plans and instrumentation and eliminates the need to remove and upsize to larger ports, which can cause unnecessary damage. Decreased tissue disruption throughout the procedure helps reduce procedural risk and can promote earlier intervention and accelerated healing.”
Cerevention is developing a portfolio of innovative neurocritical care solutions, of which the MindsEye expandable port is the first to market. “We are very excited to bring Cerevention’s innovation together with Mizuho’s strength in the neuro sales channel. This partnership will allow surgeons across the country to access this advanced technology,” said Matt Adams, Cerevention general manager.
MindsEye can be purchased by contacting Bob Todd at
[email protected] or 630-696-8209 in the Central U.S., Chris Wilson at
[email protected] or 484-683-1076 in the Eastern U.S., and Chris McCart at
[email protected] or 949-395-5422 in the Western U.S.
###
About Mizuho America
Mizuho America is a company with 30 years of history in developing the most advanced medical devices for neurosurgeons across the globe. Mizuho's state-of-the-art clips used for aneurysms and arteriovenous malformations have been a golden standard in the industry since their inception. Follow Mizuho on LinkedIn and contact the company at (510) 324-4500 or (800) 699-CLIP (2547).
About Cerevention
Cerevention, a division of Minnetronix Medical, develops neurosurgical products for sale and distribution by medical device companies. Its end-to-end services include design, development, regulatory process management, manufacturing, and commercialization services.
About Minnetronix Medical
Since 1996, Minnetronix Medical has accelerated medical device breakthroughs as a design, development, manufacturing, and commercialization partner to leading device companies around the world. Today, through lifecycle efficiency, opportunity realization, and increased utility, the company creates value in key technology segments that include RF energy, fluid and gas management, optical systems, and stimulation and active wearables. Minnetronix has the expansive industry insight and intentional technical acumen to deliver better medical devices to market, faster.