AI
-
AI firm Ketryx raises $39M, adds former Medtronic CEO as investor
The company, which helps medtech companies with AI compliance, has raised more than $55 million to date.
By Elise Reuter • Sept. 5, 2025 -
AI devices with no clinical validation tied to more recalls, study finds
Public companies, which accounted for about half of AI-enabled devices on the market, had a higher rate of recalls and a lower rate of clinical evidence, according to a JAMA study.
By Elise Reuter • Sept. 2, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtech VC funding on track to hit highest value since 2021: PitchBook
“Larger rounds increasingly favor top-tier companies and AI-native startups, leaving other startups fighting for a smaller pool of capital,” the firm said.
By Nick Paul Taylor • Sept. 2, 2025 -
Tempus inks $81M Paige buyout to support AI model development
CEO Eric Lefkofsky said that buying Paige “substantially accelerates” Tempus’ effort to build the largest foundation model in oncology.
By Nick Paul Taylor • Aug. 26, 2025 -
Philips will spend $150M to expand manufacturing for AI-enabled tech
The investment includes increasing manufacturing and research and development capabilities at its Reedsville, Pennsylvania, facility.
By Elise Reuter • Aug. 15, 2025 -
Oracle launches AI-backed EHR
The records system is available for U.S. ambulatory providers, pending regulatory approval. Oracle plans to launch in the acute care market next year.
By Emily Olsen • Aug. 14, 2025 -
Q&A
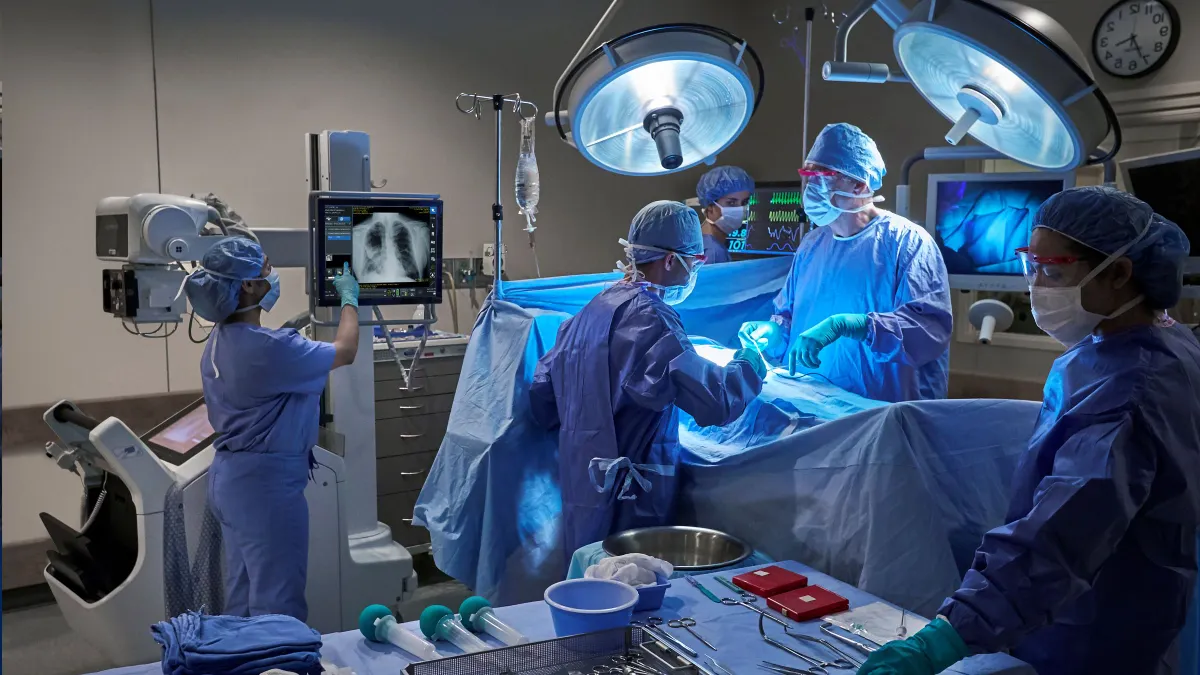
Shan Jegatheeswaran shares J&J’s vision for AI in the OR
By combining surgical video with other insights, Jegatheeswaran hopes Johnson & Johnson can support better outcomes in procedures.
By Elise Reuter • Aug. 13, 2025 -
Cardiosense appoints John Martin as chief medical officer
The appointment comes weeks after the company received 510(k) clearance for its wearable heart monitor.
By Nick Paul Taylor • Aug. 13, 2025 -
Cardiosense wins FDA clearance for wearable heart monitor
The device captures various types of heart data that could be used in AI models for cardiovascular parameters with the aim of lowering barriers to monitoring.
By Nick Paul Taylor • July 31, 2025 -
Health tech investment bolstered by AI in H1: report
Venture capital investment across the healthcare sector slowed in the first half of the year, but investors are spending on health tech — especially artificial intelligence, according to a report by Silicon Valley Bank.
By Emily Olsen • July 29, 2025 -
Trump administration releases AI adoption plan
AdvaMed CEO Scott Whitaker said the medtech lobbying group looks forward to working on policies that will unleash “the full potential of AI to help make America healthy again.”
By Emily Olsen • July 25, 2025 -
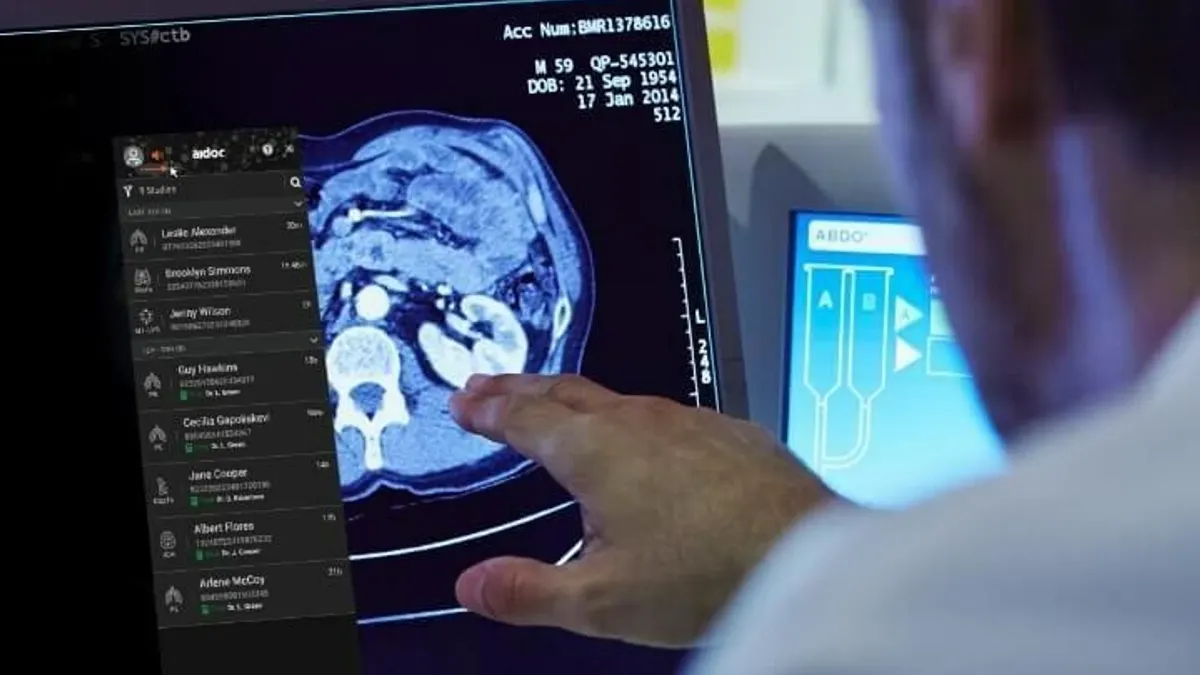
Aidoc raises $150M for AI foundation model
The technology, called CARE, is expected to allow for faster development of AI models that can cover more health conditions.
By Elise Reuter • July 24, 2025 -
Carlsmed seeks up to $103M IPO haul to fuel spine surgery growth
The company’s custom implants compete with devices from Medtronic, Johnson & Johnson and Globus Medical.
By Nick Paul Taylor • July 18, 2025 -
Q&A
Amazon’s Rowland Illing talks about AI’s shifting focus in medtech
Amazon Web Services’ global chief medical officer said the healthcare industry is seeing an “absolute explosion” of generative AI use cases, with large models that can be adapted to a variety of tasks.
By Elise Reuter • July 10, 2025 -
Danaher appoints Martin Stumpe as chief technology and AI officer
The company said Stumpe’s promotion is a “pivotal step in [its] digital transformation and its ambition to lead the next era of innovation in life sciences and diagnostics.”
By Nick Paul Taylor • June 30, 2025 -
J&J teams with Nvidia, Amazon on fund for AI surgery solutions
J&J is offering up to $100,000 in grant funding, mentorship and access to computing capabilities.
By Nick Paul Taylor • June 26, 2025 -
Joint Commission, CHAI partner to develop guidance on health AI
The guidance should help health systems safely manage and monitor AI tools in a financially sustainable manner, which can be “a real challenge” for providers, said CHAI CEO Dr. Brian Anderson.
By Emily Olsen • June 16, 2025 -
Sponsored by IQVIA MedTech
Streamlining time-to-market for AI medical devices
Learn key considerations for data selection for AI-driven MedTech development and how to identify the ideal data sets.
June 9, 2025 -
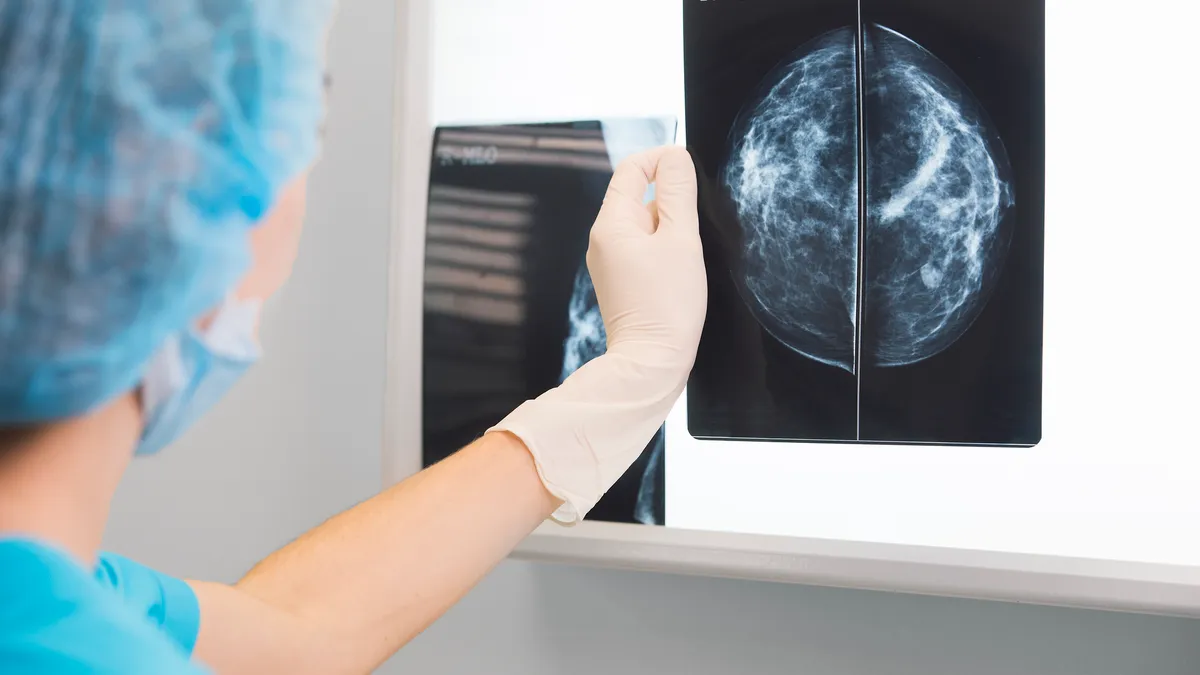
Clairity receives FDA OK for breast cancer risk prediction tool
The startup received de novo authorization for the AI tool, the first of its kind that analyzes mammogram images to predict breast cancer risk over five years.
By Elise Reuter • June 3, 2025 -
Q&A
Nvidia’s David Niewolny on the future of AI in medical devices
Nvidia, which is already working with J&J and Medtronic, plans to partner with medtech companies to build platforms for AI-enabled robotics, imaging and other features.
By Elise Reuter • June 2, 2025 -
AI in medtech is booming. Track new devices here.
The Food and Drug Administration has authorized more medical devices that incorporate artificial intelligence. Keep track of the latest developments in this database.
By Elise Reuter , Jasmine Ye Han • Updated Aug. 18, 2025 -
Deep Dive
House committees advance reconciliation text with big impacts on healthcare
Republicans in Energy and Commerce and Ways and Means have approved their portions of President Donald Trump’s “big, beautiful bill” after marathon markups. Here’s an extensive look at what the legislation means for healthcare.
By Rebecca Pifer • May 16, 2025 -
FDA, aiming to speed scientific reviews, names chief AI officer
Commissioner Martin Makary also said the agency would roll out a generative AI system across its centers that will help FDA scientists spend less time on repetitive tasks.
By Susan Kelly • May 9, 2025 -
Tracker
Trump policies are upending healthcare. Track the effect on the medtech industry here.
The FDA has rolled out an AI tool agency-wide, while a proposed HHS budget shows large cuts for the National Institutes of Health.
By MedTech Dive Staff • Updated June 6, 2025 -
Device developers advance AI plans despite Trump uncertainty
Trump administration policies have raised questions about how the FDA will regulate AI in medical devices. AdvaMed board members think the agency should keep its leading role.
By Elise Reuter • April 24, 2025