Cardiac
-
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
By Nick Paul Taylor • Aug. 8, 2025 -
Boston Scientific tells users about defibrillator problem linked to deaths, injuries
A company spokesperson said in a statement to MedTech Dive that direct causation between the deaths and calcification of the leads cannot be assumed or confirmed.
By Nick Paul Taylor • Aug. 7, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineMedical device safety in spotlight after high profile recalls
From Philips’ massive recall of respiratory devices to ongoing health risks with breast implants, medical devices tied to patient harm have put a focus on product safety.
By MedTech Dive staff -
First Corcym aortic valve implanted via new robotic procedure
CEO Christian Mazzi said the minimally invasive surgical technique could provide better outcomes for patients than transcatheter aortic valve replacement.
By Susan Kelly • Aug. 5, 2025 -
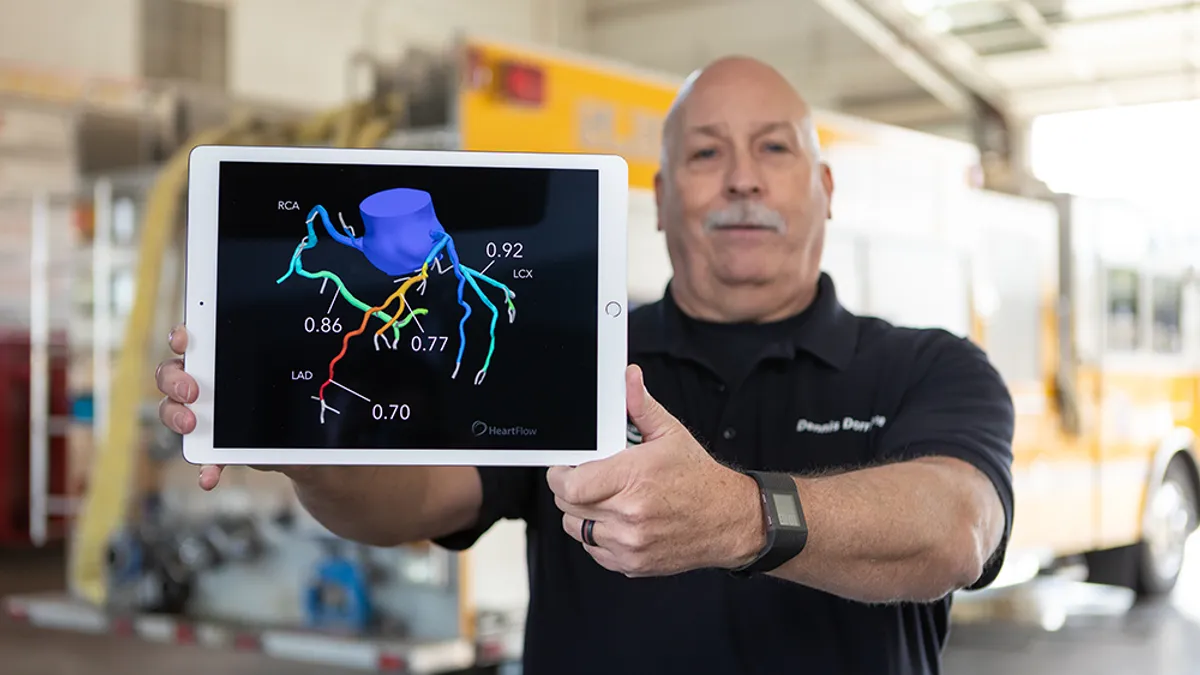
Heartflow prices IPO, aiming to net about $180M
The company, which sells software for creating 3D heart models, plans to offer 12.5 million shares at a range of $15 to $17 per share.
By Nick Paul Taylor • Aug. 4, 2025 -
News roundup
Monogram robot marks autonomy milestone; Stereotaxis catheter cleared
Monogram Technologies conducted its first fully autonomous robotic knee replacement surgery, while Stereotaxis won FDA clearance for a robotically navigated electrophysiology mapping catheter.
By Susan Kelly • July 31, 2025 -
Cardiosense wins FDA clearance for wearable heart monitor
The device captures various types of heart data that could be used in AI models for cardiovascular parameters with the aim of lowering barriers to monitoring.
By Nick Paul Taylor • July 31, 2025 -
Edwards loses longtime TAVR leader Larry Wood
Wood, who helped build Edwards’ TAVR business, will become CEO of Procept BioRobotics. Edwards also raised its sales and earnings outlook for the full year.
By Susan Kelly • July 25, 2025 -
Edwards recalls arterial cannulas over exposed wires
Exposed wires could puncture the artery and cause bleeding, inadequate perfusion and hemolysis, the FDA said in its Class I recall notice.
By Nick Paul Taylor • July 25, 2025 -
Heartflow files for IPO
The company reported revenue of $37.2 million in the first quarter, up 39% year over year, but said it has faced challenges in achieving higher rates of adoption in the past.
By Nick Paul Taylor • July 21, 2025 -
Earnings & Tariffs
‘We are not rolling over’: J&J electrophysiology unit rebounds amid PFA rivalry
The division returned to growth in the second quarter after declining over the previous three months. Now, can the healthcare giant catch up with its rivals in pulsed field ablation?
By Ricky Zipp • July 16, 2025 -
Abbott gains national CMS coverage for tricuspid valve repair
The agency’s decision supports broader access to a minimally invasive treatment for patients with severe tricuspid regurgitation, in which blood can leak backward into the heart.
By Susan Kelly • July 10, 2025 -
Boston Scientific gets expanded PFA label for persistent AFib
Patients whose atrial fibrillation lasts longer than seven days and who do not respond well to drug therapy are now eligible for treatment with the Farapulse pulsed field ablation system.
By Susan Kelly • July 8, 2025 -
J&J’s Abiomed recalls heart pump controllers after 3 patients die
The FDA published an early alert, which the agency reserves for potentially high-risk issues.
By Nick Paul Taylor • Updated July 8, 2025 -
Kardium pulls in an additional $250M to advance PFA tech
The funding will allow the Canadian company to pursue regulatory approvals and build up its manufacturing capabilities and commercial team ahead of a launch anticipated this year.
By Susan Kelly • July 7, 2025 -
Field Medical raises another $35M for PFA system
The medtech startup founded by physician and Farapulse inventor Steven Mickelsen is preparing to begin a pivotal study of pulsed field ablation for ventricular tachycardia.
By Susan Kelly • July 2, 2025 -
Carmat files for insolvency amid cash crunch
The artificial heart maker ran a crowdfunding campaign to try to raise some of the 3.5 million euros it needed to fund operations into July.
By Nick Paul Taylor • July 1, 2025 -
Bivacor wins FDA breakthrough nod for titanium total artificial heart
The company has so far treated patients who were weeks or months away from a transplant, but the device is theoretically suitable for longer-term use.
By Nick Paul Taylor • June 2, 2025 -
Boston Scientific plans changes to expected $200M tariff hit as situation shifts
The company also shared more details of its exit from the TAVR market and the impact of FDA layoffs on product review times.
By Nick Paul Taylor • May 29, 2025 -
Boston Scientific ends TAVR sales; J&J shares Monarch robot data
Boston Scientific said it would halt worldwide sales of its Acurate devices due to “increased clinical and regulatory requirements.”
By Elise Reuter • May 28, 2025 -
Edwards gains expanded TAVR label for asymptomatic patients
The medical technology company expects the new indication to increase sales in its transcatheter aortic valve replacement business with more patients becoming eligible for the treatment.
By Susan Kelly • May 5, 2025 -
Medtronic wins FDA approval for defibrillation lead
Omniasecure, which was authorized for placement in the heart’s right ventricle, is the world’s smallest defibrillation lead, according to Medtronic.
By Nick Paul Taylor • Updated May 5, 2025 -
News roundup
4 PFA studies in the spotlight at Heart Rhythm 2025
Boston Scientific, Medtronic, Abbott and J&J unveiled new data for their PFA devices in tandem with the Heart Rhythm Society’s annual meeting.
By Susan Kelly • April 29, 2025 -
Earnings & Tariffs
Edwards shrugs off tariffs, backs 2025 profit forecast
The heart valve specialist saw better-than-expected TAVR sales in the first quarter as hospitals addressed capacity constraints that have dogged its core business.
By Susan Kelly • Updated April 30, 2025 -
Field Medical raises $40M for PFA in ventricular tachycardia
Led by Farapulse founder Steven Mickelsen, Field has developed a pulsed field ablation device to tackle the dangerous heart rhythm disorder.
By Susan Kelly • April 23, 2025 -
Earnings & Tariffs
Boston Scientific is ‘very bullish’ on 2025 despite $200M tariff hit
The company joins Johnson & Johnson and Abbott as top medtech firms that expect an impact in the hundreds of millions of dollars from President Donald Trump’s tariff policies.
By Ricky Zipp • April 23, 2025