Clinical Trials
-
3 medtech takeaways from ESC 2025
Pulsed field ablation and transcatheter aortic valve implants were in focus at the European Society of Cardiology Congress in Madrid.
By Susan Kelly • Sept. 3, 2025 -
Vicarious Surgical delays robot timeline
Days into the job, CEO Stephen From said the company no longer expects to begin a clinical trial for its robotic system by the end of the year.
By Susan Kelly • Aug. 13, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
News roundup
Monogram robot marks autonomy milestone; Stereotaxis catheter cleared
Monogram Technologies conducted its first fully autonomous robotic knee replacement surgery, while Stereotaxis won FDA clearance for a robotically navigated electrophysiology mapping catheter.
By Susan Kelly • July 31, 2025 -
Freenome posts pivotal liquid biopsy data as FDA filing nears completion
Freenome plans to complete all modules of its premarket approval submission in mid-2025.
By Nick Paul Taylor • June 4, 2025 -
AstraZeneca drug could help keep a common breast cancer at bay
Data presented at ASCO show that swapping in the oral drug camizestrant for an older therapy helped sustain the benefit of initial treatment, potentially opening a novel step in patient care.
By Ned Pagliarulo • June 2, 2025 -
Teal posts data on newly approved at-home cancer screening device
Self-collection correctly identified 95.2% of HPV samples that are associated with increased cancer risk.
By Nick Paul Taylor • May 20, 2025 -
News roundup
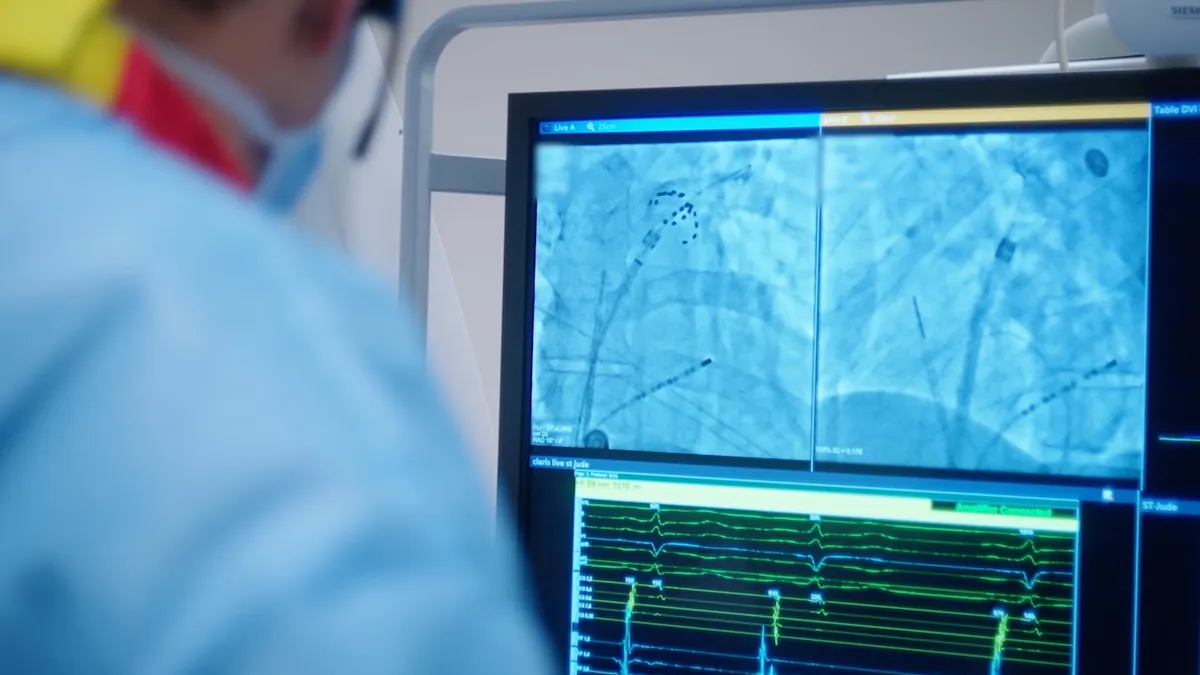
4 PFA studies in the spotlight at Heart Rhythm 2025
Boston Scientific, Medtronic, Abbott and J&J unveiled new data for their PFA devices in tandem with the Heart Rhythm Society’s annual meeting.
By Susan Kelly • April 29, 2025 -
J&J completes first surgeries with Ottava robot
Johnson & Johnson plans to seek a U.S. indication for multiple general surgery procedures, including gastric bypass, which was performed in the clinical trial.
By Susan Kelly • April 15, 2025 -
Sponsored by Danaher
Innovating a healthier tomorrow: Entering a new era of medicine
Explore the cutting-edge of science and learn how Danaher is advancing healthcare innovation.
April 14, 2025 -
J&J starts IVL trial in ‘difficult-to-cross’ coronary arteries
The device is differentiated from other Shockwave products, and rival catheters from Abbott and Boston Scientific, because it emits waves from its tip.
By Nick Paul Taylor • April 8, 2025 -
Boston Scientific’s Farapulse matches Medtronic cryoablation in trial
Atrial tachyarrhythmia recurred in 39 patients treated with Boston Scientific’s Farapulse PFA device, while 53 people had recurrence in the Medtronic cryoablation group.
By Nick Paul Taylor • April 3, 2025 -
3 takeaways from ACC 2025
Medtronic, Abbott and Boston Scientific had updates on their devices used in heart valve disease treatment at the event.
By Susan Kelly • April 2, 2025 -
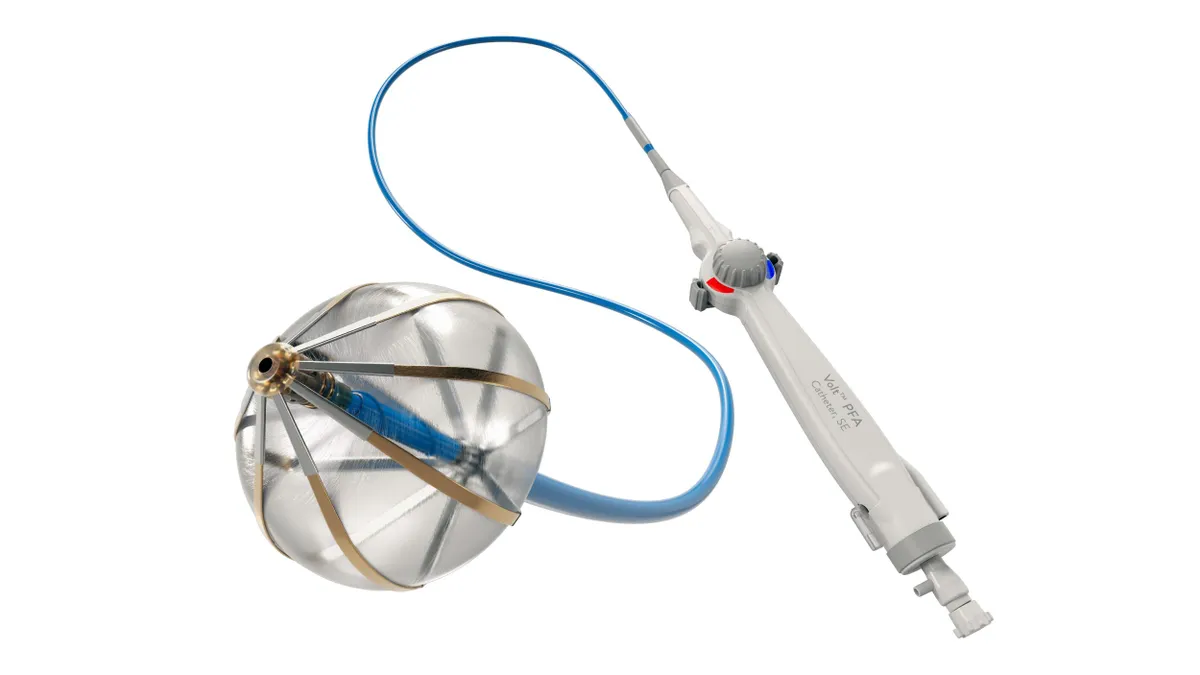
Abbott snags early CE mark for Volt PFA device
With PFA becoming physicians’ preferred ablation method for treating AFib, Abbott is pushing to catch up with rival systems already on the market.
By Susan Kelly • March 28, 2025 -
Academic medical centers say funding cuts jeopardize health research
Proposed cuts to National Institutes of Health funding threaten a critical pipeline of innovation and discovery necessary to fuel advancements in patient care, according to researchers and provider groups.
By Susanna Vogel • March 25, 2025 -
Abbott gets FDA nod to begin IVL trial after rivals buy up competition
J&J acquired the IVL device maker Shockwave Medical last year for $13.1 billion, and Boston Scientific agreed to buy Bolt Medical in January for up to $664 million.
By Nick Paul Taylor • March 25, 2025 -
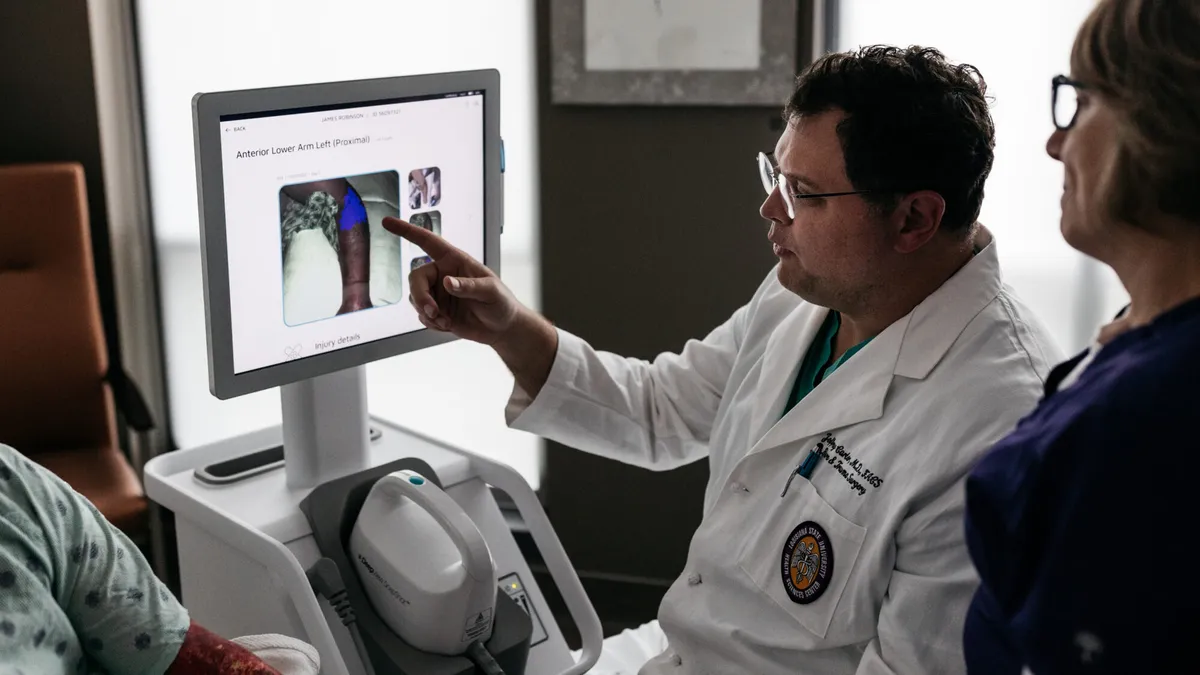
 Retrieved from Spectral AI on March 18, 2025
Retrieved from Spectral AI on March 18, 2025
Spectral AI algorithms beat physicians in burn tissue assessments
The company plans to file the results with the FDA by mid-2025 and launch the product in 2026.
By Nick Paul Taylor • March 18, 2025 -
Capstan brings surgical robot to valve replacement
Capstan Medical, a startup focused on robotic-assisted mitral and tricuspid valve replacement, recently completed its first patient implants.
By Susan Kelly • March 11, 2025 -
EMA creates advice portal for some high-risk medical devices
The European Medicines Agency said the portal will enable consultations with medical device expert panels that can promote faster access to new technologies.
By Nick Paul Taylor • Feb. 12, 2025 -
 Retrieved from Screenshot: The Food and Drug Administration.
Retrieved from Screenshot: The Food and Drug Administration.
FDA webpages on clinical trial diversity removed after Trump orders
The website changes raise concerns about “the interference of politics with the study and the practice” of science and medicine, a physician said.
By Elise Reuter • Jan. 27, 2025 -
Medtronic links closed-loop pain device to improvements after 12 months
The Inceptiv data give Medtronic more evidence as it tries to increase sales of a product that drove double-digit growth at the company’s pain stimulation unit last quarter.
By Nick Paul Taylor • Jan. 24, 2025 -
‘Research that’s desperately needed’: White House conference spotlights women’s health
“Sometimes the most under-hyped sectors are the best places to build very large businesses,” said one venture investor who attended the Dec. 11 event.
By Delilah Alvarado • Dec. 19, 2024 -
Movano receives FDA nod for smart ring’s pulse oximeter feature
Movano Health plans to market the Evie Ring to organizations that run clinical trials and healthcare companies that are helping patients manage chronic diseases.
By Nick Paul Taylor • Dec. 3, 2024 -
Medtronic, Tempus testing AI to find potential TAVR patients
The randomized trial will use Tempus’ platform to find patients who are eligible for interventions such as TAVR but do not have a treatment plan in place.
By Nick Paul Taylor • Nov. 25, 2024 -
Boston Scientific’s Watchman could be new option for patients post ablation: study
The trial compared the stroke prevention device for left atrial appendage closure to blood thinners in people who underwent cardiac ablation for AFib.
By Susan Kelly • Nov. 18, 2024 -


Photo by MART PRODUCTION from Pexels

Livanova hits sleep apnea trial goals, plans FDA filing
Leerink analysts said the results were largely in line with outcomes in a trial of Inspire Medical’s rival device but cautioned that Livanova may have a hard time breaking into the sleep apnea market.
By Nick Paul Taylor • Nov. 13, 2024