COVID-19: Page 17
-
Abbott, BD, Quidel tests get EUAs for asymptomatic COVID-19 serial screening
While the Abbott and Quidel authorizations are for over-the-counter use, BD's test requires a prescription, which "along with the fact an instrument is needed, limits the use-cases for the company significantly," William Blair analysts wrote.
By Greg Slabodkin • April 1, 2021 -
Quest sells minority share of Q2 Solutions for $760M
IQVIA, which previously owned 60% of Q2 Solutions, will now become the sole owner of the clinical trials laboratory company. Quest and IQVIA established Q2 Solutions as a joint venture in 2015.
By Ricky Zipp • April 1, 2021 -
A year into the pandemic, advanced cancer diagnoses are rising
Research from radiation oncologists and molecular pathologists add to evidence showing that many people skipped getting cancer screenings in 2020 as they avoided going to the doctor to minimize the risk of COVID-19 infection.
By Susan Kelly • March 31, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
FDA updates impact of mutations on COVID-19 tests, adds Cepheid to list of affected products
A new agency webpage features the latest information about the potential impact of viral mutations on molecular diagnostics such as Cepheid's Xpert line of rapid SARS-CoV-2 tests.
By Nick Paul Taylor • March 31, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
Analysts tip surgeries to rebound. Rising COVID-19 cases threaten those forecasts.
Two Wall Street reviews suggest people are visiting hospitals more and are increasingly comfortable with undergoing elective care, but they come amid the CDC chief's warning that rising hospitalizations may jeopardize progress.
By Nick Paul Taylor • March 30, 2021 -
Abbott, Quest, Roche among cos pitching K-12 COVID-19 test plan to tap into Biden's $10B fund
A who's who of sector companies are teaming with the Rockefeller Foundation on the proposal. The industry stands to benefit from widespread school testing as symptomatic demand diminishes.
By Nick Paul Taylor • March 26, 2021 -
Hospitals flailed amid COVID-19 crisis, are unsure of future, OIG says
Executives said they were worried about their workers experiencing trauma and concerned a shrinking recruitment pool for nurses could exacerbate staffing shortages.
By Ron Shinkman • March 25, 2021 -
Thermo Fisher pivots COVID-19 approach with air detection, school testing
The company is looking to new strategies as vaccines drive down cases and institutions like schools, hospitals and businesses re-open.
By Nick Paul Taylor • March 25, 2021 -
Roche bets COVID-19 vaccination drive to favor PCR over antigen testing
Wall Street analysts contend the Swiss diagnostic company's argument could bode well for Abbott, Hologic, Thermo Fisher Scientific and PerkinElmer.
By Nick Paul Taylor • March 24, 2021 -
Cancer screenings bounced back after steep pandemic declines, Rand report shows
The rate of women seeking mammograms was higher by the end of July than in the months leading up to the pandemic, though colonoscopies did not return to pre-pandemic levels, researchers found.
By Samantha Liss • March 23, 2021 -
AstraZeneca, Oxford vaccine prevents COVID-19 in big US study amid controversy overseas
A two-shot regimen was 79% effective at protecting people from COVID-19, which should support the fourth clearance of a vaccine in the U.S. and calm safety concerns abroad.
By Ben Fidler • March 22, 2021 -
FDA grants EUA to COVID-19 screening device using machine learning
While the product gives institutions another screening tool for those without symptoms, Tiger Tech faces competition with antigen testing scaling up.
By Nick Paul Taylor • March 22, 2021 -
EU group proposes minimum standards for rapid antibody COVID-19 tests
Antibody tests of questionable accuracy proliferated on both sides of the Atlantic early in the pandemic, leading the U.S. and European Union to try to raise standards.
By Nick Paul Taylor • March 19, 2021 -
ASCs gave medtechs alternate care sites amid pandemic hospital elective shutdown
After surgeries moved to ambulatory surgery centers during the pandemic's upending of non-emergency care, experts and industry believe some procedures may never go back.
By Ricky Zipp • March 19, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
FDA's 1st full OK to BioFire's COVID-19 test clears shorter path for others
Full agency approval of the diagnostic, now permitted to be marketed beyond the public health emergency, lets other companies secure non-emergency authorizations through a 510(k).
By Nick Paul Taylor • March 19, 2021 -
Abbott awarded $255M federal contract for rapid COVID-19 antigen tests
The company will initially provide 50 million of its point-of-care diagnostics to HHS for use in Florida and Maine, with the potential for up to $766 million under the eight-month deal.
By Greg Slabodkin • March 18, 2021 -
Deep Dive
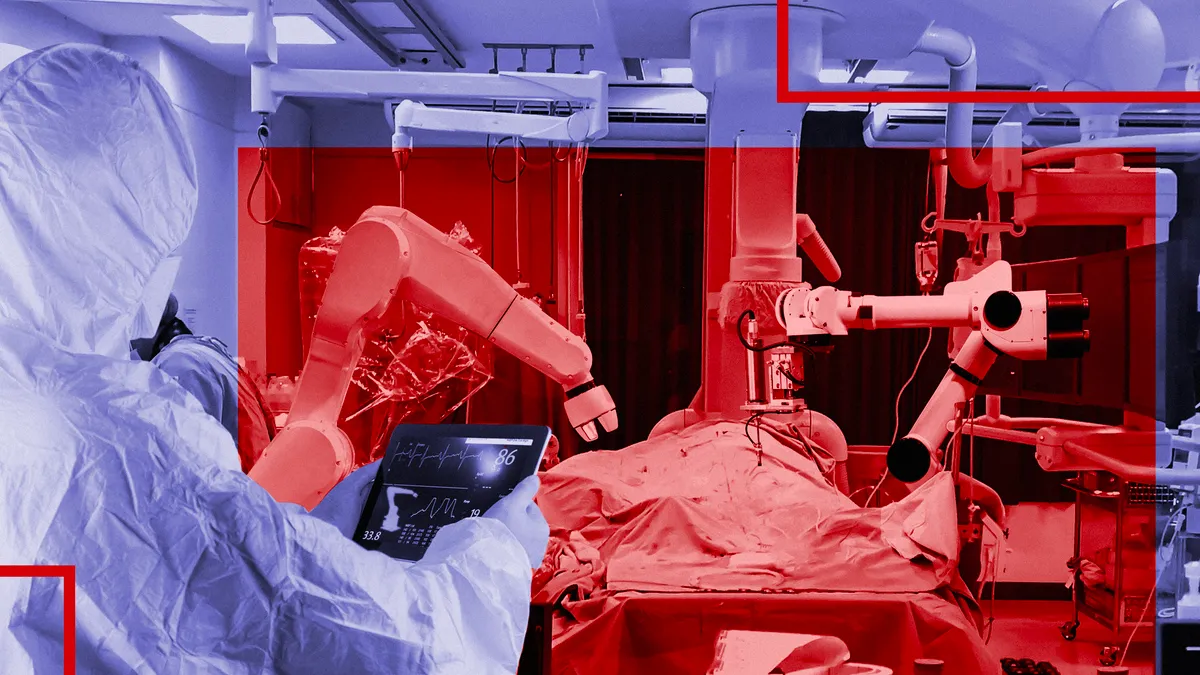
Robotics demand held firm as pandemic pummeled electives
Intuitive Surgical, Stryker and Zimmer Biomet all saw procedure volumes drop in 2020 as electives shut down. However, system demand remained strong despite the financial hit to hospitals.
By Ricky Zipp • March 18, 2021 -

 Retrieved from Official White House Photo by Adam Schultz.
Retrieved from Official White House Photo by Adam Schultz.
Biden commits $12B for COVID-19 testing to reopen schools, address disparities
While not identifying company recipients, the administration called out both PCR tests that deliver results within 24 hours and point-of-care antigen kits as suitable for school screening programs.
By Nick Paul Taylor • March 18, 2021 -
Deep Dive
From labs to homes: where COVID-19 testing is headed in 2021
The U.S. appears to have reached a new phase in its battle against the coronavirus with vaccinations taking precedence over tests. Whether testing will return to earlier levels is an open question.
By Greg Slabodkin • March 17, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
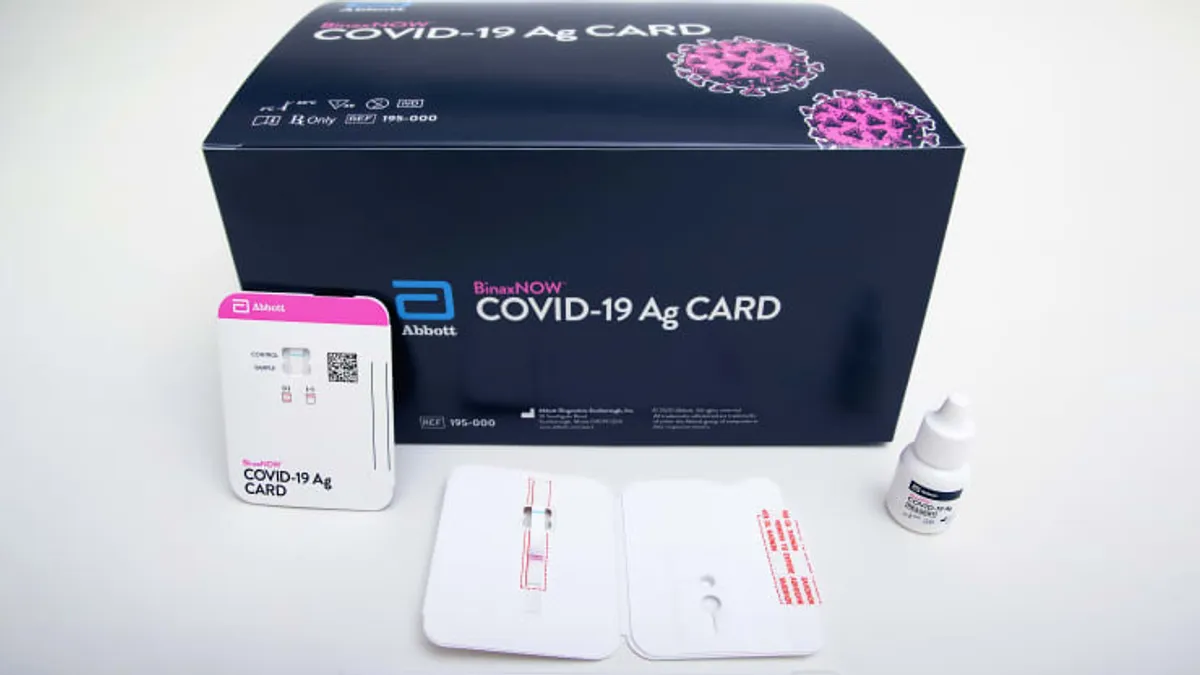
FDA backs home use of COVID-19 tests without data in asymptomatic people
The move could benefit companies like Quidel, which aims to market an over-the-counter antigen test but has been held up by the need for more data on those without symptoms.
By Nick Paul Taylor • March 17, 2021 -
Deep Dive
All eyes on elective care after a rollercoaster year for medtech
After shutdowns slammed procedure-dependent firms in 2020, industry and Wall Street are waiting to see when non-emergency surgeries return and what a comeback might look like.
By Ricky Zipp • March 16, 2021 -
1 year after COVID-19 hit: what's next for FDA, electives, testing and robotics
The medtech industry has ridden a roller coaster of steep demand for novel diagnostics and a plunge in once-stable business lines like hip and knee replacement surgeries.
March 15, 2021 -
Roche to buy GenMark for $1.8B to add multiplex testing
The Swiss company said the deal will give it access to a novel technology for testing a range of pathogens with one patient sample. William Blair analysts expect "potentially intense bidding wars" in the sector in coming months.
By Greg Slabodkin • March 15, 2021 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration
 Deep Dive
Deep Dive5 things medtech can expect from FDA in 2021
"What you saw under the prior administration was this concept of a kinder, softer FDA to industry," said Dennis Gucciardo, partner at Morgan Lewis. Experts now expect a shift, including more enforcement activity.
By Greg Slabodkin • March 15, 2021 -
FDA flags false positives from Roche's COVID-19-flu test
The agency is asking users who suspect their systems are suffering from either issue identified by the Swiss medtech giant to stop running tests on them and contact the company.
By Nick Paul Taylor • March 15, 2021