Diabetes
-
Insulet promotes Eric Benjamin to chief operating officer
As new CEO Ashley McEvoy lays the groundwork to build the brand globally, the diabetes tech company also hired Manoj Raghunandanan as chief growth officer.
By Elise Reuter • Aug. 26, 2025 -

 Retrieved from Luna Health on August 20, 2025
Retrieved from Luna Health on August 20, 2025
Luna Health raises $23.6M for tiny insulin patch pump
The startup intends to make a small patch pump with a closed-loop algorithm for nighttime glucose control.
By Elise Reuter • Aug. 20, 2025 -
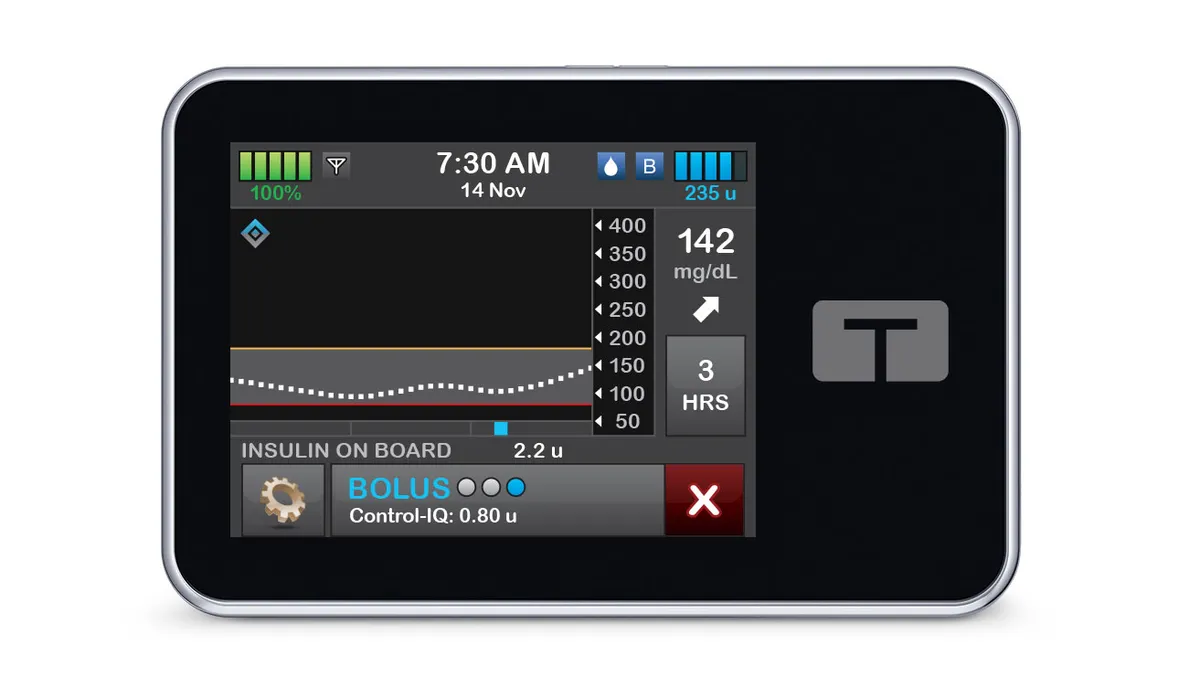
Tandem, Insulet monitoring CMS payment proposal for diabetes tech
The proposal would introduce competitive bidding for insulin pumps and change how Medicare pays for diabetes devices, if finalized.
By Elise Reuter • Aug. 8, 2025 -
Tandem insulin pump malfunction linked to 59 injuries
A problem with speakers in Tandem’s t:slim X2 insulin pumps can cause insulin delivery to stop, the company said.
By Elise Reuter • Aug. 7, 2025 -
Dexcom raises sales expectations, discusses G8 plans
Outgoing CEO Kevin Sayer expects expanding coverage of Dexcom’s glucose sensors for people with Type 2 diabetes and over-the-counter devices to drive future growth.
By Elise Reuter • July 31, 2025 -
Dexcom CEO Kevin Sayer to step down
Jake Leach will take over the role at the start of 2026, with Sayer remaining chairman of the board after the transition.
By Elise Reuter • Updated July 31, 2025 -
Medtronic receives Type 2 label expansion for MiniMed 780G in Europe
The CE mark also allows Medtronic’s automated insulin delivery system to be used during pregnancy and by children as young as 2.
By Elise Reuter • July 23, 2025 -
Earnings & Tariffs
Abbott lowers sales forecast on diagnostics decline, US funding cuts
Medical device sales remained a bright spot for the company, however, with more than 20% growth in diabetes devices.
By Elise Reuter • July 17, 2025 -
Medtronic names CFO for diabetes spinoff
The company named Chad Spooner CFO of MiniMed ahead of plans to spin out the diabetes unit into a separate public firm.
By Elise Reuter • July 8, 2025 -
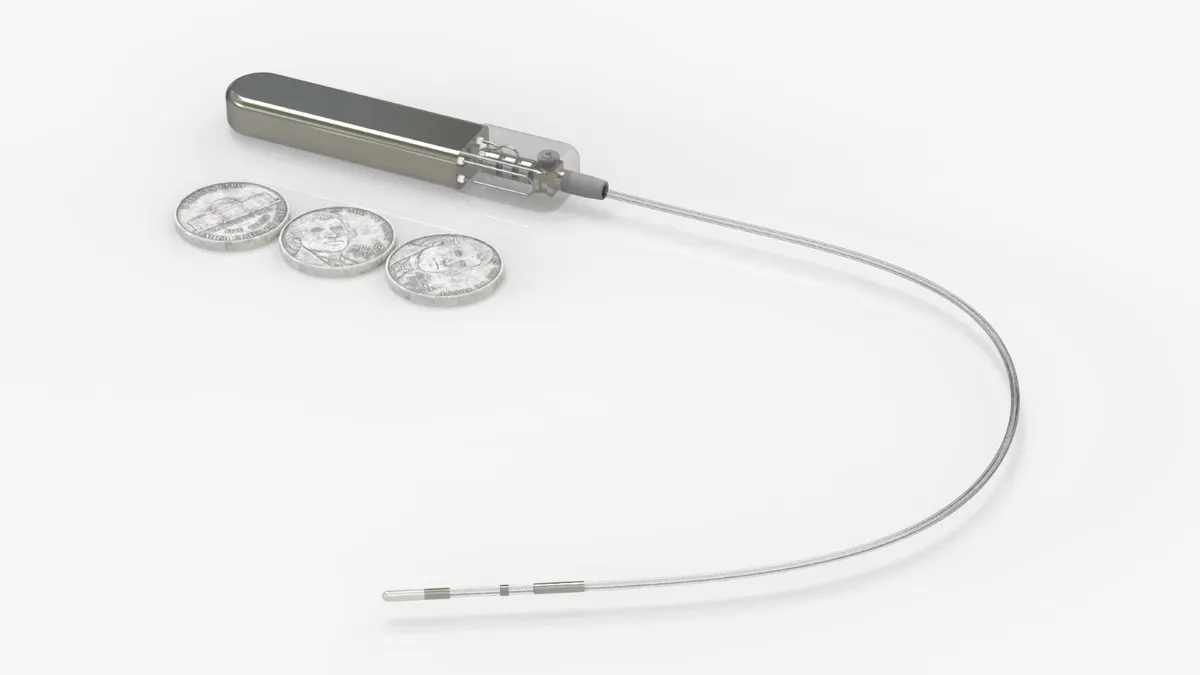
Implantable glucose monitor competitor emerges with early data
Glucotrack, which is developing a glucose monitor that is implanted through a minimally invasive surgery, said the small study met safety and performance goals.
By Elise Reuter • July 8, 2025 -
Who benefits from over-the-counter CGMs?
Experts speaking at the ADA’s Scientific Sessions supported making CGMs more accessible, but debated their usefulness for adults without diabetes.
By Elise Reuter • July 1, 2025 -
New glucose sensors, insulin patch pumps lead ADA’s Scientific Sessions
The diabetes conference featured a debate about over-the-counter glucose monitors and updates on upcoming devices, including insulin patch pumps. Here’s a roundup of MedTech Dive’s coverage.
By Elise Reuter • July 1, 2025 -
Tandem’s Elizabeth Gasser on patch pump plans, Abbott partnership
The strategy and product exec said Tandem aims to give patients options and reach more people who haven’t yet used insulin pumps.
By Elise Reuter • June 25, 2025 -
ADA takeaways: Diabetes tech firms preview new patch pumps, glucose sensors
Medtronic, Beta Bionics and Tandem Diabetes Care showcased planned patch pumps at the conference, while Senseonics shared updates on its implantable glucose sensors.
By Elise Reuter • June 24, 2025 -
Insulet’s Eric Benjamin shares update on automated insulin delivery system
The chief product and customer experience officer said Insulet has seen an uptick in users with Type 2 diabetes since receiving an expanded indication, and shared an update on plans for a closed-loop system.
By Elise Reuter • June 23, 2025 -
5 things to watch at the ADA’s Scientific Sessions
The American Diabetes Association’s annual conference kicks off amid a dynamic year for diabetes tech, as access improves for people with Type 2 diabetes and companies debut new devices.
By Elise Reuter • June 20, 2025 -
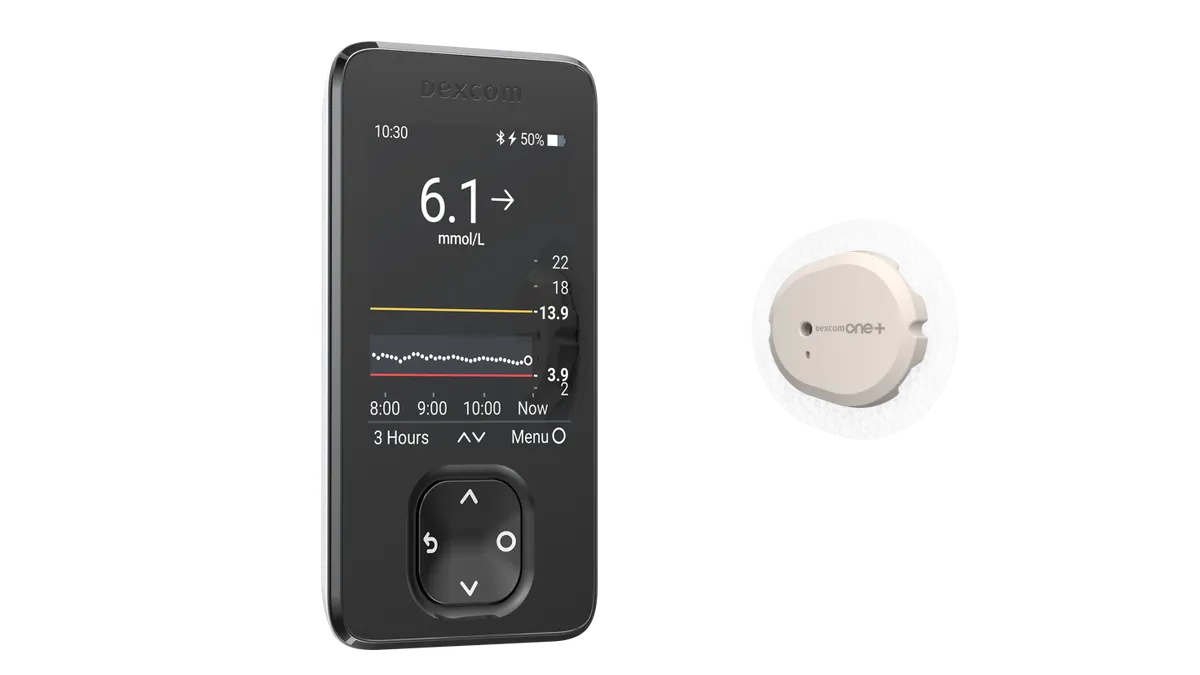
Dexcom recalls more than 700,000 CGM receivers for lack of audible alarm
Severe adverse events, including seizure and loss of consciousness, were potentially linked to the issue, based on 56 reports Dexcom received.
By Elise Reuter • Updated June 24, 2025 -
Q&A
Abbott’s new diabetes leader on latest sensors, partnerships and Type 2 coverage
Ahead of the American Diabetes Association’s Scientific Sessions, Chris Scoggins said Abbott’s ambition is to be the most broadly connected diabetes tech company.
By Elise Reuter • June 12, 2025 -
Tandem, Abbott team on insulin delivery with glucose-ketone sensor
Tandem will integrate its insulin delivery systems with a new dual glucose-ketone sensor being developed by Abbott.
By Elise Reuter • June 10, 2025 -
Owens & Minor spikes $1.36B Rotech buyout over regulatory barriers
The companies scrapped the merger because securing FTC clearance “proved unviable in terms of time, expense and opportunity.”
By Nick Paul Taylor • June 6, 2025 -
Q&A
Beta Bionics CEO, CFO on how to take a company public
CEO Sean Saint and CFO Stephen Feider spoke about what’s different now that the company is public and the long journey of pursuing an IPO.
By Ricky Zipp • June 2, 2025 -
Why Medtronic plans to spin out its diabetes business
While some analysts questioned why Medtronic would leave a fast-growing market, others backed the company’s plan to focus on segments with higher margins.
By Elise Reuter • May 22, 2025 -
Medtronic plans to spin off diabetes business within 18 months
CEO Geoff Martha told investors the split simplifies Medtronic’s portfolio, while creating a stand-alone diabetes tech competitor.
By Ricky Zipp • May 21, 2025 -
Dexcom CEO says CGMs fit MAHA agenda ‘very nicely’; Tandem preps for new products
In earnings calls, diabetes tech companies had less to say about tariffs and focused more on future growth.
By Elise Reuter • May 16, 2025 -
Embecta restructures again, outlines tariff costs
The diabetes tech company announced a second restructuring after ending its insulin patch-pump program.
By Elise Reuter • May 13, 2025