Diabetes: Page 4
-
Embecta gets FDA nod for insulin patch pump
Embecta’s first patch pump features a larger insulin reservoir, based on feedback from people with Type 2 diabetes.
By Elise Reuter • Sept. 3, 2024 -
Q&A
Insulet’s Trang Ly on overcoming ‘clinical inertia’ in diabetes care
Automated insulin delivery for Type 2 patients can help change minds among physicians who have been reluctant to prescribe pump therapy, the chief medical officer said in an interview.
By Susan Kelly • Aug. 27, 2024 -
Insulet expands Omnipod 5 pump to people with Type 2 diabetes
The FDA clearance marks the first automated insulin delivery system for people with Type 2 diabetes.
By Elise Reuter • Aug. 26, 2024 -
Dexcom prices first over-the-counter glucose monitor
Known as Stelo, the device is intended for people who don’t take insulin and costs $89 per month.
By Elise Reuter • Aug. 26, 2024 -
Tandem warns iPhone app still draining insulin pump batteries
Despite a software update in March, users are still reporting that the app is rapidly depleting their insulin pump batteries, causing unexpected shutdowns.
By Elise Reuter • Aug. 15, 2024 -
Abbott, Medtronic partner on diabetes tech
Abbott will develop a glucose sensor that can only connect with Medtronic's insulin delivery technology.
By Elise Reuter • Aug. 7, 2024 -
Q&A
Ascensia’s new head of CGM talks plans for a 365-day sensor
Brian Hansen recently joined Ascensia, which markets Senseonics’ implantable glucose sensors. The current version lasts 180 days, but Senseonics is seeking FDA approval for a one-year sensor.
By Elise Reuter • Aug. 6, 2024 -
Patient shares a day in the life with diabetes at FDA’s first Home Health Hub meeting
The initiative, led by new CDRH Acting Director Michelle Tarver, is intended to improve health equity by including people’s living conditions in device design.
By Elise Reuter • July 30, 2024 -
Q&A
Tandem CMO shares Mobi feedback, expansion plans
MedTech Dive caught up with Chief Medical Officer Jordan Pinsker to talk about early feedback on Tandem’s smallest insulin pump and what’s next for the device.
By Elise Reuter • July 29, 2024 -
Dexcom shares plunge on lower sales outlook
“I’m just kind of in shock,” an analyst said on the earnings call, asking for more detail on the forecast cut.
By Elise Reuter • July 26, 2024 -
Abbott recalls Freestyle Libre 3 sensors due to incorrect glucose readings
A spokesperson for Abbott said the recall “may impact less than 1% of Libre 3 users in the U.S.”
By Nick Paul Taylor • July 26, 2024 -
Diabetes device firm Embecta considers sale: Financial Times
Two years after Embecta spun out of BD, the firm is now exploring a potential sale, the Financial Times reported.
By Elise Reuter • July 22, 2024 -
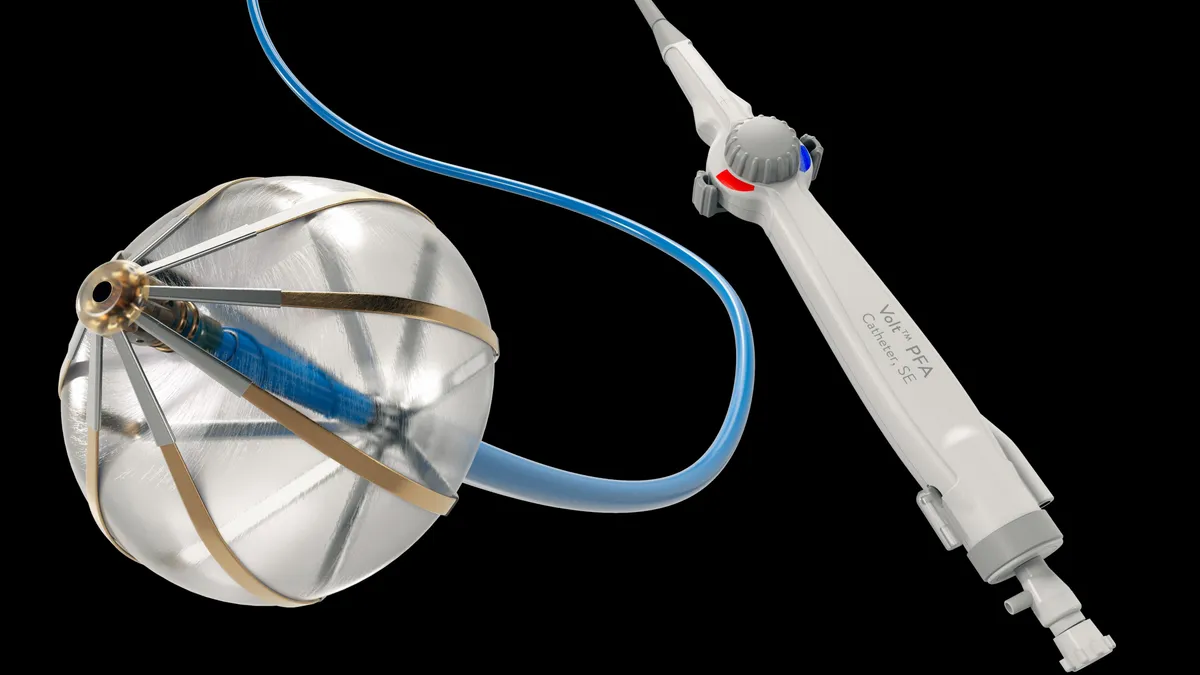
Abbott previews PFA, over-the-counter CGM markets
Electrophysiology sales held up against rivals’ launches of pulsed field ablation systems. Meanwhile, Abbott is gauging sales of its first OTC CGMs.
By Elise Reuter • July 18, 2024 -
J&J, Abbott and Intuitive start medtech earnings season
The companies’ financial results will offer a glimpse at the current health of medtech markets.
By Nick Paul Taylor • July 16, 2024 -
Roche wins CE mark for its first CGM
The clearance positions Roche to challenge Abbott and Dexcom for the European continuous glucose monitor market.
By Nick Paul Taylor • Updated July 10, 2024 -
Abbott and Dexcom are launching the first over-the-counter CGMs. Here are 7 questions on the new tech.
Experts expect the release of new over-the-counter glucose monitors in the U.S. to fuel more widespread use of the devices.
By Elise Reuter • June 26, 2024 -
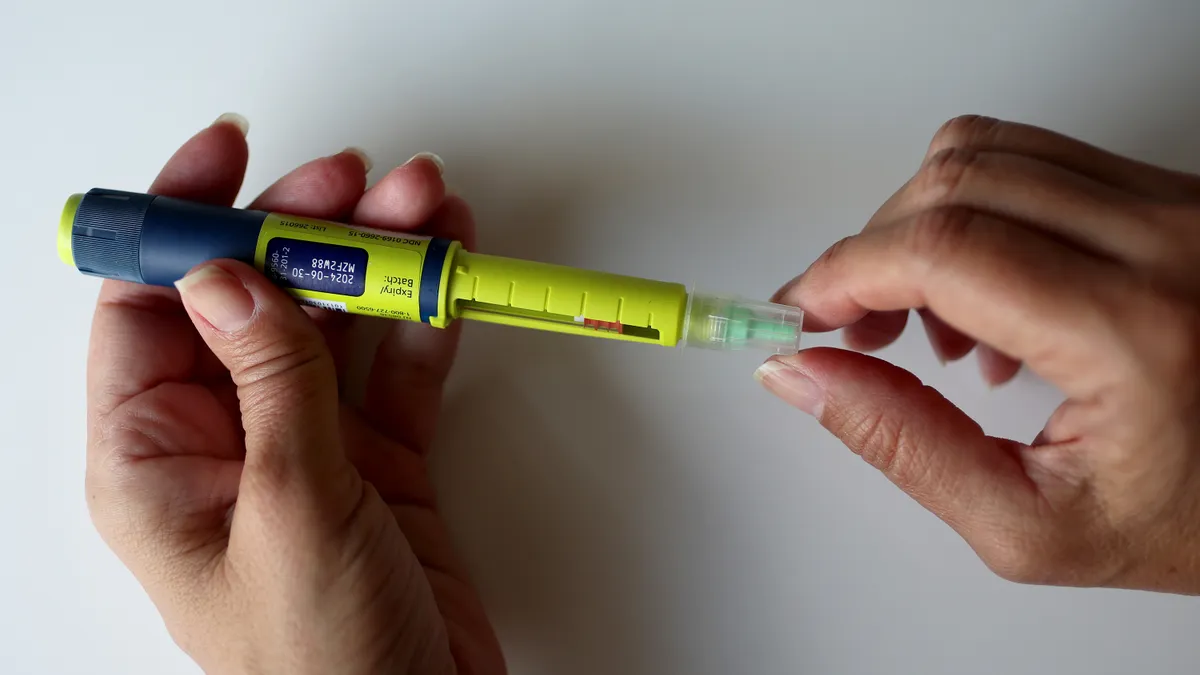
Embecta touts its insulin patch pump at ADA
Embecta said its device, with a larger insulin reservoir, addresses an unmet need for people with Type 2 diabetes.
By Elise Reuter • June 25, 2024 -

 Retrieved from Ascensia Diabetes Care on June 25, 2024
Retrieved from Ascensia Diabetes Care on June 25, 2024
Senseonics tips 365-day CGM to double 2025 sales
Senseonics is in talks with insulin pump manufacturers about integrating its implantable Eversense continuous glucose monitor with their devices.
By Nick Paul Taylor • June 25, 2024 -
Insulet seeks label expansion of Omnipod 5 for Type 2 diabetes
At the American Diabetes Association meeting, Insulet shared study results showing improvements in blood glucose and quality of life.
By Elise Reuter • June 24, 2024 -
Tandem names Jean-Claude Kyrillos as chief operating officer
The appointment gives Kyrillos a leading role in work to expand the insulin delivery company’s global operations and achieve profitable growth.
By Nick Paul Taylor • June 21, 2024 -
Abbott ramps up over-the-counter CGM race with new sensors
Abbott will launch two over-the-counter glucose monitors in the U.S., competing directly with Dexcom’s Stelo.
By Elise Reuter • June 10, 2024 -

 Retrieved from Dexcom on June 05, 2024
Retrieved from Dexcom on June 05, 2024
Dexcom connects G7 CGM directly to Apple Watch in US
Apple Watch and G7 users can now view real-time glucose readings on their wrist, regardless of whether they are carrying an iPhone.
By Nick Paul Taylor • Updated June 7, 2024 -
Abbott receives FDA OK for over-the-counter glucose monitor
After debuting its Lingo device in the U.K. last year, Abbott can now sell the sensor in the U.S.
By Elise Reuter • June 3, 2024 -
Medicare adviser sets recommendations for diabetes device evidence
A MEDCAC panel found time in range was an “extremely important” metric, but members were divided on whether quality of life measures should influence coverage.
By Elise Reuter • May 22, 2024 -
Dexcom details plans for over-the-counter CGM as insulin pump firms seek Type 2 coverage
Diabetes tech companies shared updates on new products, largely focused on Type 2 patients, in Q1 earnings calls.
By Elise Reuter • May 16, 2024