Diagnostics: Page 33
-
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
FDA updates impact of mutations on COVID-19 tests, adds Cepheid to list of affected products
A new agency webpage features the latest information about the potential impact of viral mutations on molecular diagnostics such as Cepheid's Xpert line of rapid SARS-CoV-2 tests.
By Nick Paul Taylor • March 31, 2021 -
FTC challenges Illumina-Grail deal, putting $7B merger in jeopardy
The commission said the union will "harm competition in the U.S. market for life-saving multi-cancer early detection tests." Illumina is opposing the action, but Wall Street analysts see no "easy fixes."
By Nick Paul Taylor • March 31, 2021 -
Abbott, Quest, Roche among cos pitching K-12 COVID-19 test plan to tap into Biden's $10B fund
A who's who of sector companies are teaming with the Rockefeller Foundation on the proposal. The industry stands to benefit from widespread school testing as symptomatic demand diminishes.
By Nick Paul Taylor • March 26, 2021 -
Thermo Fisher pivots COVID-19 approach with air detection, school testing
The company is looking to new strategies as vaccines drive down cases and institutions like schools, hospitals and businesses re-open.
By Nick Paul Taylor • March 25, 2021 -
Roche bets COVID-19 vaccination drive to favor PCR over antigen testing
Wall Street analysts contend the Swiss diagnostic company's argument could bode well for Abbott, Hologic, Thermo Fisher Scientific and PerkinElmer.
By Nick Paul Taylor • March 24, 2021 -
Cancer screenings bounced back after steep pandemic declines, Rand report shows
The rate of women seeking mammograms was higher by the end of July than in the months leading up to the pandemic, though colonoscopies did not return to pre-pandemic levels, researchers found.
By Samantha Liss • March 23, 2021 -
EU group proposes minimum standards for rapid antibody COVID-19 tests
Antibody tests of questionable accuracy proliferated on both sides of the Atlantic early in the pandemic, leading the U.S. and European Union to try to raise standards.
By Nick Paul Taylor • March 19, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
FDA's 1st full OK to BioFire's COVID-19 test clears shorter path for others
Full agency approval of the diagnostic, now permitted to be marketed beyond the public health emergency, lets other companies secure non-emergency authorizations through a 510(k).
By Nick Paul Taylor • March 19, 2021 -
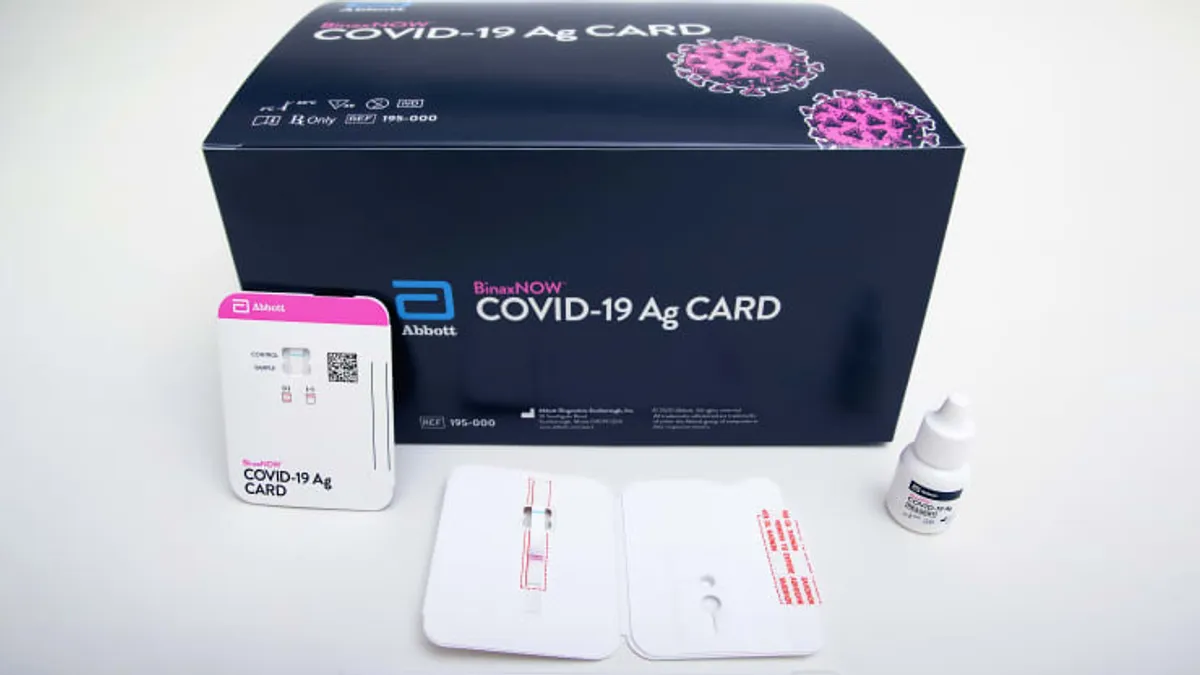
Abbott awarded $255M federal contract for rapid COVID-19 antigen tests
The company will initially provide 50 million of its point-of-care diagnostics to HHS for use in Florida and Maine, with the potential for up to $766 million under the eight-month deal.
By Greg Slabodkin • March 18, 2021 -

 Retrieved from Official White House Photo by Adam Schultz.
Retrieved from Official White House Photo by Adam Schultz.
Biden commits $12B for COVID-19 testing to reopen schools, address disparities
While not identifying company recipients, the administration called out both PCR tests that deliver results within 24 hours and point-of-care antigen kits as suitable for school screening programs.
By Nick Paul Taylor • March 18, 2021 -
Deep Dive
From labs to homes: where COVID-19 testing is headed in 2021
The U.S. appears to have reached a new phase in its battle against the coronavirus with vaccinations taking precedence over tests. Whether testing will return to earlier levels is an open question.
By Greg Slabodkin • March 17, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
FDA backs home use of COVID-19 tests without data in asymptomatic people
The move could benefit companies like Quidel, which aims to market an over-the-counter antigen test but has been held up by the need for more data on those without symptoms.
By Nick Paul Taylor • March 17, 2021 -
FDA touts real-world evidence use by Abbott, Medtronic in analysis of regulatory decisions
The agency has released details of how medtechs have used RWE, such as registries and medical records, to support filings including 510(k) submissions and premarket approval applications.
By Nick Paul Taylor • March 17, 2021 -
1 year after COVID-19 hit: what's next for FDA, electives, testing and robotics
The medtech industry has ridden a roller coaster of steep demand for novel diagnostics and a plunge in once-stable business lines like hip and knee replacement surgeries.
March 15, 2021 -
Roche to buy GenMark for $1.8B to add multiplex testing
The Swiss company said the deal will give it access to a novel technology for testing a range of pathogens with one patient sample. William Blair analysts expect "potentially intense bidding wars" in the sector in coming months.
By Greg Slabodkin • March 15, 2021 -
Deep Dive
5 things medtech can expect from FDA in 2021
"What you saw under the prior administration was this concept of a kinder, softer FDA to industry," said Dennis Gucciardo, partner at Morgan Lewis. Experts now expect a shift, including more enforcement activity.
By Greg Slabodkin • March 15, 2021 -
FDA flags false positives from Roche's COVID-19-flu test
The agency is asking users who suspect their systems are suffering from either issue identified by the Swiss medtech giant to stop running tests on them and contact the company.
By Nick Paul Taylor • March 15, 2021 -
Quest eyes $2B DTC testing potential to capitalize on 'breakout' consumer growth
The lab giant's next move is to start selling an $89 at-home fecal test, targeting the colorectal cancer screening market led by Exact Sciences' Cologuard.
By Nick Paul Taylor • March 12, 2021 -
Quidel says COVID-19 test demand is plunging. Wall Street is underwhelmed.
CEO Doug Bryant said demand plummeted between 30% and 40% in February and March compared to the fourth quarter, sending shares down 17% on Wednesday.
By Greg Slabodkin • March 11, 2021 -
GE Healthcare predicts growth, hike in digital investment as pandemic eases
"We are seeing procedure volumes increasing, contributing to the rebound of our pharmaceutical diagnostics business and we see sequential market growth in imaging and ultrasound," CEO Kieran Murphy said during an investor event.
By Nick Paul Taylor • March 11, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
Thermo Fisher, PerkinElmer broaden COVID-19 mutation tracking products
The companies will sell PCR assays to labs to enable surveillance, coming amid warnings that the U.S. is "flying blind" when it comes to tracking rapidly emerging SARS-CoV-2 variants.
By Greg Slabodkin • March 10, 2021 -
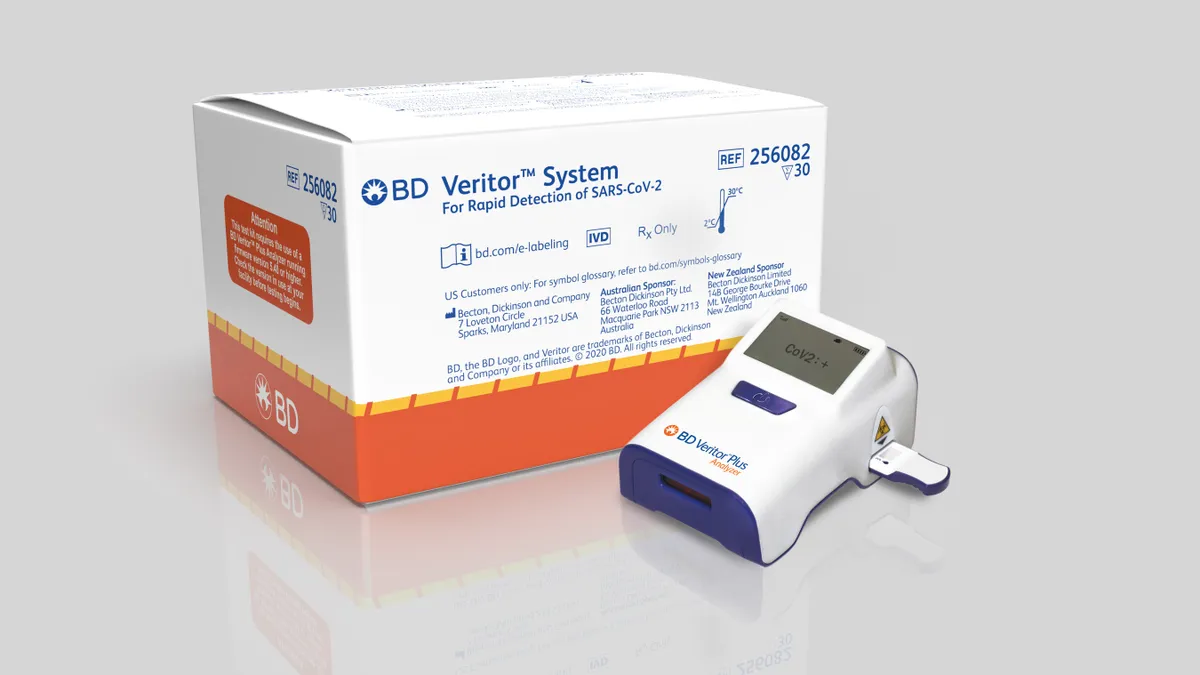
BD, Abbott using apps to ease COVID-19 antigen test data bottlenecks
Both companies are using apps to capture and store testing data with public health agencies. The U.S. government has invested hundreds of millions of dollars to increase antigen testing, but data sharing hurdles remain.
By Nick Paul Taylor • March 10, 2021 -
EU remote audits under MDR in doubt as divergent national positions persist
Team-NB pulled a proposal unveiled in February amid sustained resistance. A Commission medical device group met last week but has yet to disclose a solution.
By Nick Paul Taylor • March 10, 2021 -
Coronavirus relief bill with rural hospital aid, over $47B for testing passes Senate
The nearly $2 trillion bill includes $47.8 billion to boost COVID-19 testing and contact tracing efforts, including supporting the development, manufacturing and administering of tests.
By Shannon Muchmore • March 8, 2021 -
FDA grants first EUA to at-home OTC molecular test for COVID-19
Cue Health received the emergency authorization and expects to be able to produce more than 100,000 tests a day by the summer, as the agency continues to prioritize more at-home testing options.
By Nick Paul Taylor • March 8, 2021