Diagnostics: Page 42
-
Guardant growth slows in Q2 but still beats expectations
The precision oncology company reported a 23% increase in sales, yet refrained from reinstating full-year guidance amid ongoing uncertainty about the impact of COVID-19.
By Nick Paul Taylor • Aug. 7, 2020 -
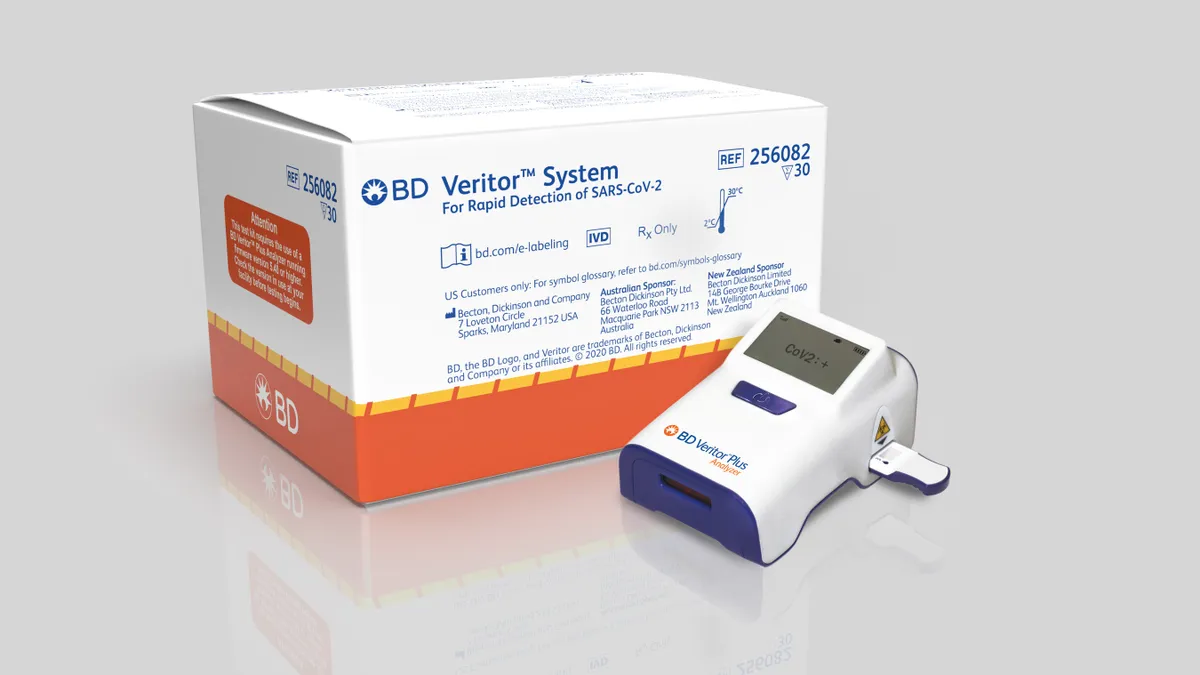
BD sales slide below expectations, as COVID-19 antigen test gains momentum
In the month since the launch of its antigen test, CEO Tom Polen said BD shipped more Veritor readers than in a typical year. Still, the medtech's stock fell 9% Thursday amid pressure on other business lines.
By Greg Slabodkin • Aug. 6, 2020 -
10 states now on board to buy Quidel, BD antigen tests
With the latest additions of Arkansas and Rhode Island, there are now five Democratic and five Republican governors each planning to acquire half a million of the rapid point-of-care diagnostics from the medtechs.
By Greg Slabodkin • Updated Aug. 19, 2020 -
Americas drag on Qiagen as Thermo Fisher deal goes down to the wire
A hedge fund that owns 8% of Qiagen "fully" expects the Thermo Fisher offer to fail, calling it “wholly inadequate.”
By Nick Paul Taylor • Aug. 5, 2020 -
CMS proposes eliminating inpatient-only list, in potential boon for ASCs
The agency would begin by removing about 300 musculoskeletal-related services, which could further accelerate ambulatory surgery centers' important role as medtech customers.
By Shannon Muchmore • Aug. 4, 2020 -
Device maker Q2 reports show optimism on COVID-19 recovery, but flare-ups keep guidance at bay
Since March and April lows, business volume has improved sequentially. Still, many medtechs are tightlipped on what that may mean for financial performance for the rest of the year.
By Maria Rachal • Aug. 3, 2020 -
Quidel says COVID-19 antigen test buoys 270% rise in rapid diagnostics unit, demand 'more than we can satisfy'
The company late Thursday reported second quarter total sales of $201.8 million, an 86% increase over the prior year, with strong demand for its PCR and antigen tests for the coronavirus.
By Greg Slabodkin • Updated July 31, 2020 -
Exact Sciences bets pandemic will accelerate adoption of Cologuard
The cancer diagnostics company is spending to drive a lasting move from colonoscopies to its at-home screening test, including running a $70 million TV advertising campaign.
By Nick Paul Taylor • July 31, 2020 -
Hologic blows away earnings expectations on COVID-19 testing surge
The manufacturer said it has ramped production capacity to at least 1.5 million coronavirus tests a week, estimating it provided a quarter to a third of the test results delivered in the U.S. during the recent quarter.
By Susan Kelly • July 30, 2020 -
Coronavirus could fuel clinical lab M&A by stressing smaller hospital players
Consultancy Kaufman Hall predicts a ramp-up of deals throughout the year, as hospital-owned labs seek support amid the pandemic. That forecast meshes with comments from Quest's CEO last week.
By Nick Paul Taylor • July 29, 2020 -
Hologic wins HHS-DOD contract to boost COVID-19 test supplies production
Some analysts peg Hologic's current share of the U.S. COVID-19 molecular testing market between 25% and 30% and predict the manufacturing expansion could create a $150 million windfall in quarterly revenue.
By Susan Kelly • July 28, 2020 -
LabCorp says COVID-19 testing 'more than' offsets still-recovering base business
LabCorp's improving average turnaround time for reporting molecular diagnostic test results stands in contrast to Quest, which for most of July has reported average result wait times for non-priority patients of a week or more.
By Greg Slabodkin • July 28, 2020 -
Color targets supply, staffing bottlenecks with updated coronavirus test EUA
The population health technology company's test uses dry nasal swabs, eliminating the need for the viral transport media that's been in short supply and limited scaling by lab giants Quest and LabCorp.
By Nick Paul Taylor • July 28, 2020 -
Deep Dive
Coronavirus testing hits a wall: Where do we go from here?
As LabCorp and Quest are backlogged in delivering molecular test results, some public health officials are calling for a shift in testing approach from slow and accurate to fast and good enough to meet demand.
By Greg Slabodkin • July 27, 2020 -
Medtech and COVID-19: 6 months into the public health emergency
MedTech Dive examines where the industry stands with testing and the future of those emergency use-authorized diagnostics, as well as how it's adapting to a rebound in elective surgeries and various supply chain challenges.
July 27, 2020 -
How COVID-19 is challenging and changing medtech supply chain management
Four experts share insights on the supply and demand hangups medtechs have been working through during the pandemic, and a few early takeaways the industry can carry forward.
By Maria Rachal • July 27, 2020 -
Diagnostics makers must plan for EUA products to have post-pandemic future
An extension to the public health emergency formalized a reprieve for emergency use authorizations, but manufacturers should act now if they want products to stay on the market when the crisis ends, compliance experts say.
By Greg Slabodkin • July 27, 2020 -
LabCorp COVID-19 test gets 1st FDA OK for asymptomatic screening
U.S. regulators simultaneously allowed the LabCorp test to be used in sample pooling, following the first such nod to a Quest Diagnostics test a week ago.
By Nick Paul Taylor • July 27, 2020 -
COVID-19 diagnostics emerging as prime investment target, SVB analysis shows
Companies with tech involved in the pandemic response raised $1.3 billion over the first half of the year, with developers of tests and R&D tools enjoying a 62% increase in investment.
By Nick Paul Taylor • July 24, 2020 -
HHS renews COVID-19 public health emergency
The extension supported by AdvaMed, the American Clinical Laboratory Association and American Hospital Association takes effect Saturday and opens the door for FDA to keep issuing emergency use authorizations.
By Shannon Muchmore • July 23, 2020 -
COVID-19 test demand backs 61% jump in Roche molecular diagnostic sales in 2020
"As far as the molecular diagnostics business is concerned, there is no doubt we will sell anything we can produce over the next half year,” Roche CEO Severin Schwan said on an earnings call with investors.
By Nick Paul Taylor • July 23, 2020 -
Quest says COVID-19 hotspot spikes starting to hit routine testing recovery
In addition to soaring demand for coronavirus testing, the lab giant's Q2 was helped by gradual improvement in base testing volumes. But recent outbreaks have sent numbers down again in July.
By Greg Slabodkin • July 23, 2020 -
Medical device deal values down 88% amid COVID-19 disruption: PwC
Going forward, M&A may rise in light of recent deferral of elective procedures, which analysts say “could create the need for significant consolidation as companies work to remain competitive.”
By Nick Paul Taylor • July 23, 2020 -
Thermo Fisher rakes in $1.3B in coronavirus-related Q2 revenue
The company expects momentum to continue in the third quarter after posting springtime results showing a more than 70% jump in its diagnostic and healthcare end market sales fueled by soaring demand for COVID-19 testing.
By Nick Paul Taylor • July 22, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
AdvaMed launches COVID-19 test registry to address shortages
Created in partnership with Abbott, Roche, Siemens and others, the database is aimed at helping ensure sufficient testing supply by providing weekly state- and national-level updates on the number of tests shipped.
By Nick Paul Taylor • July 22, 2020