Diagnostics: Page 49
-
 U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
FDA chief warns of supply 'pressure' on reagents for coronavirus tests
Qiagen, a major supplier of RNA extraction kits, confirmed to MedTech Dive Thursday that "extraordinary demand for coronavirus testing workflows" is challenging the company's capacity.
By Greg Slabodkin • March 12, 2020 -

 U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
White House, CDC under fire for availability of coronavirus tests
The Trump administration is facing criticism that federal agencies did not engage the big private labs early enough in the public health emergency.
By Greg Slabodkin • March 11, 2020 -
ECRI: Poor sterilization, failure to learn from device problems threaten patient safety
The 2020 list put together by the nonprofit ECRI Institute also highlights diagnostic errors as a top patient safety concern for a third consecutive year.
By Maria Rachal • March 10, 2020 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
Labs step up capacity to meet demand for nationwide coronavirus testing
The Trump administration is relying on the "enormous capacity" of commercial labs to enable wide availability of COVID-19 diagnostic testing to the American public through physicians offices and pharmacies.
By Greg Slabodkin • March 10, 2020 -
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
ACC 2020 meeting canceled as coronavirus spreads in US
Abbott, Edwards and Medtronic were among the medtechs slated to present data at the American College of Cardiology's annual event. "This is a unique time for us all," the organization wrote in a statement Monday.
By Maria Rachal • March 9, 2020 -

 CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
$8.3B in coronavirus funding set in motion as federal agencies ramp up response
The House and Senate passed a bill with allocations for medical supplies and testing as Vice President Mike Pence met with clinical lab execs Wednesday from LabCorp, Quest Diagnostics, Thermo Fisher and others.
By Shannon Muchmore • Updated March 5, 2020 -
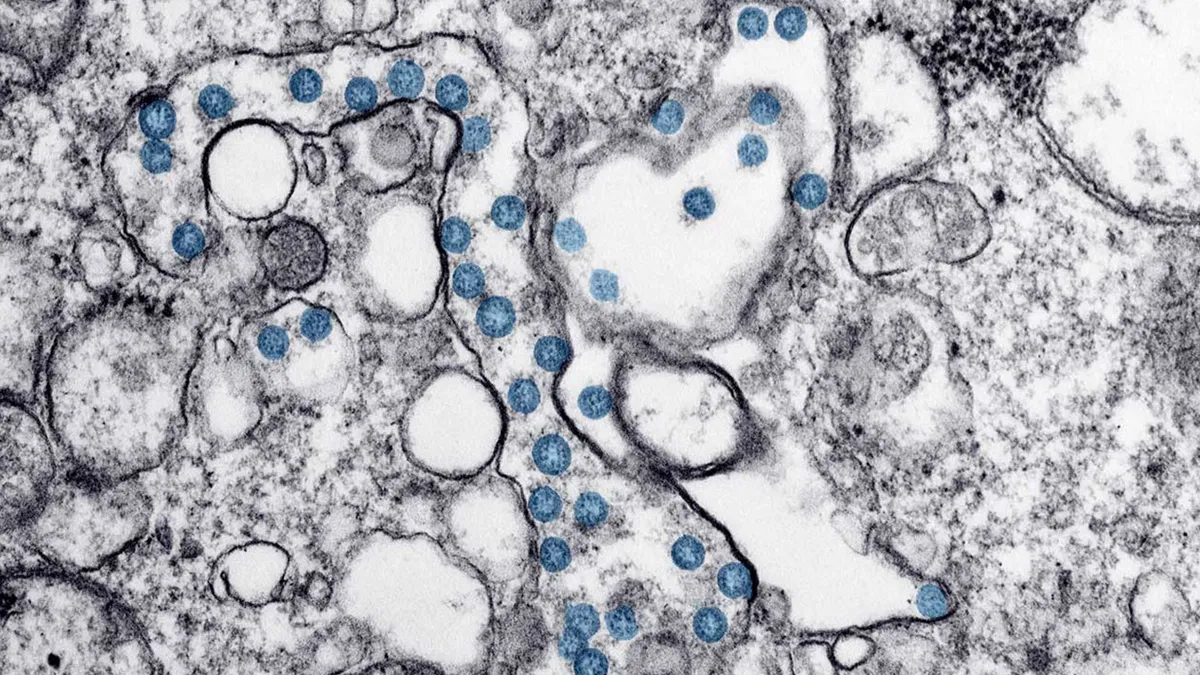
 CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
LabCorp launches coronavirus test, Quest in the wings
The updates from the major U.S. clinical labs come days after FDA OK'ed the use of lab developed tests that have not yet received emergency use authorization.
By Maria Rachal • March 5, 2020 -
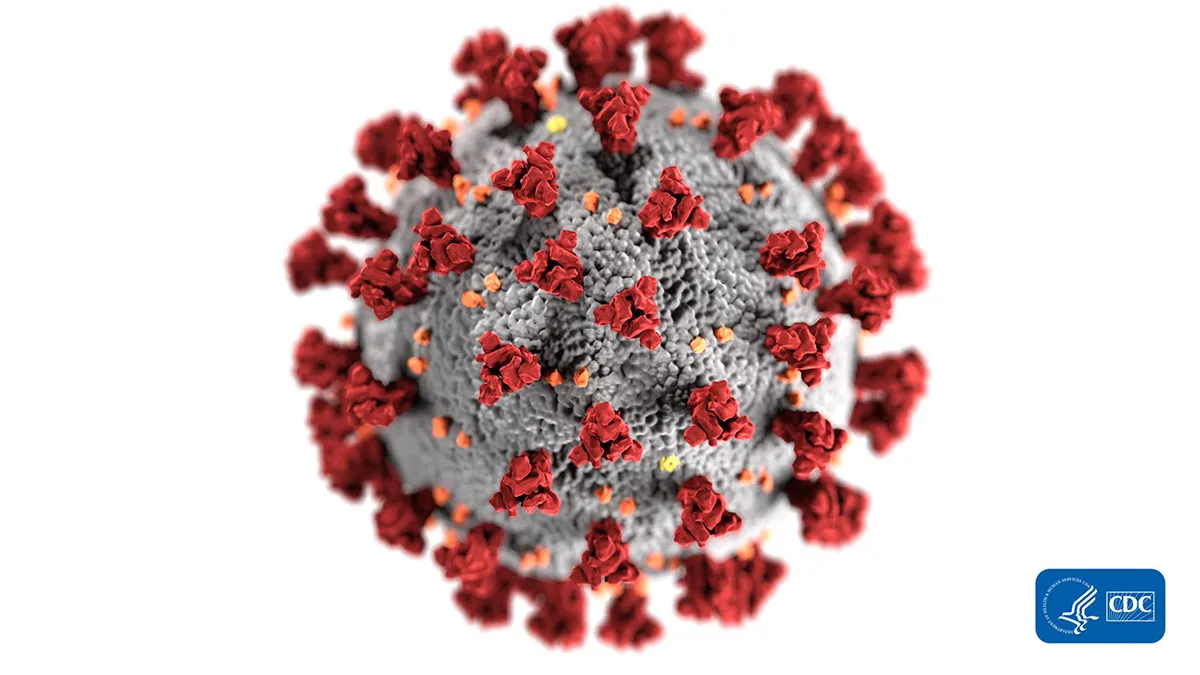
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
Insurer lobby urges coronavirus test coverage, but can't mandate changes
“They can’t require member companies to do anything,” said Sabrina Corlette, a research professor at Georgetown University's Center on Health Insurance Reforms.
By Samantha Liss • March 5, 2020 -
UK looks to use Brexit to make device industry more transparent
The House of Commons bill seeks to address a regulatory gap for medical devices, as the previous legal framework is based on EU directives. One element provides new information-sharing powers related to safety.
By Nick Paul Taylor • March 5, 2020 -
Exact Sciences closes takeovers of 2 cancer testing businesses
The deals give the diagnostics company a solid tumor test and R&D capabilities as it aims to expand beyond its flagship Cologuard product.
By Nick Paul Taylor • March 4, 2020 -
After waffling, Qiagen agrees to $11.5B buyout by Thermo Fisher
Months of will-they-won't-they M&A rumors culminated in the Dutch test maker accepting an offer for a 23% premium on its shares, as Thermo Fisher aims to strengthen its molecular diagnostics and infectious disease efforts.
By Maria Rachal • March 3, 2020 -
FDA seeks 'right balance' as it permits immediate use of coronavirus tests
"We are not changing our standards for issuing Emergency Use Authorizations," Commissioner Stephen Hahn said Saturday as the agency issued new guidance aimed at accelerating testing capacity in the U.S.
By Susan Kelly • March 2, 2020 -
Coronavirus: No US shortages of essential devices yet, as FDA monitors more than 60 companies
Still, manufacturers operating 72 plants in China face workforce pressures and surging demand for some equipment, FDA said in an update late Thursday.
By Nick Paul Taylor • Feb. 28, 2020 -
FDA pilots new 510(k) submission template for device manufacturers
The voluntary assessment will enroll up to nine participants that reflect the makeup of the medical device industry.
By Nick Paul Taylor • Feb. 27, 2020 -
FDA bolsters CLIA waiver application recommendations
The agency added new sections on failure alerts, labeling and safeguards, doubling the length of a guidance text for in vitro diagnostic manufacturers in the process.
By Nick Paul Taylor • Feb. 26, 2020 -
Guardant predicts net loss doubling as it takes on Exact Sciences' Cologuard
The cancer testing company's CEO said new studies could open up significant market opportunities, justifying the spending surge.
By Nick Paul Taylor • Feb. 25, 2020 -
1st test for genetic condition tied to developmental delay wins FDA nod
Austin, Texas-based biotech Asuragen won De Novo authorization for a blood test for Fragile X Syndrome, which the agency said is the most common known cause of inherited developmental delay and intellectual disability.
By Maria Rachal • Feb. 24, 2020 -
FDA tracks uptick in masks, other protective gear in coronavirus update
Commissioner Stephen Hahn said the shift in ordering patterns is yet to manifest in a shortage but warned Friday the situation is “evolving and very dynamic.”
By Nick Paul Taylor • Feb. 18, 2020 -
LabCorp weathers rising personnel costs to beat expectations in Q4
Quest Diagnostics said the trend is an emerging source of pressure, while LabCorp had a more tempered reaction.
By Nick Paul Taylor • Feb. 13, 2020 -
Exact Sciences beefs up sales force as it seeks big Cologuard growth in 2020
The cancer diagnostics company said fresh TV ads and physicians' increasing ability to place orders through an updated version of Epic will spur sales of its colorectal cancer test.
By Nick Paul Taylor • Updated March 3, 2020 -
Snapshot: How medtech earnings season is shaping up
Most medtechs offered financial outlooks for 2020 over the last month, but the novel coronavirus could have a longer impact than originally factored in.
By Maria Rachal • Updated March 6, 2020 -
Longtime Myriad CEO resigns amid another steep earnings miss, guidance cut
Sales in the prenatal and hereditary cancer divisions, plus the seemingly promising GeneSight line, all declined during the quarter.
By Nick Paul Taylor • Feb. 7, 2020 -
Qiagen, still independent, limps back from 2019
The Dutch maker of infectious disease instruments and consumables is seeking a rebound, with an early boost from coronavirus-driven demand. Cowen analysts noted the firm beat expectations but added: "The bar was quite low."
By Nick Paul Taylor • Feb. 5, 2020 -
Emergency use coronavirus tests shows glitches at state level, CDC says
CDC officials said Wednesday that some of the test kits it distributed to states produced inconclusive results when tested independently. The agency noted the tests were not run on patients.
By Maria Rachal • Updated Feb. 13, 2020 -
Brexit happened. EU MDR looms. 3 key questions on medtech's future in Europe
The U.K. can skip the new in vitro diagnostic rules entirely, and in theory, could unilaterally stop applying MDR after the transition period ends. Both scenarios seem unlikely.
By Nick Paul Taylor • Feb. 4, 2020