Medical Devices
-
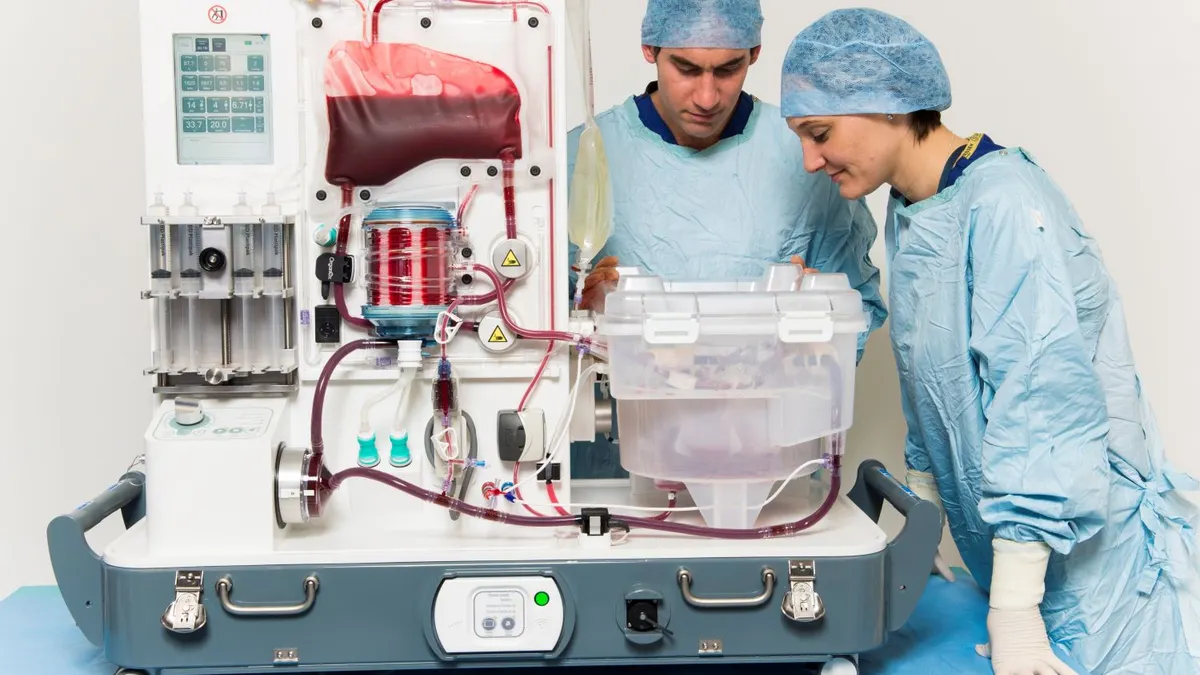
Terumo to buy OrganOx for $1.5B to enter transplant field
OrganOx could capture a greater share of the liver transplant market, where it competes with TransMedics Group, Needham analyst Mike Matson wrote.
By Susan Kelly • Aug. 25, 2025 -
Medtech firms shuffle execs in 2025
Top leaders at Intuitive, Boston Scientific, Dexcom and others changed roles this year. Read MedTech Dive’s roundup for all the details.
By Elise Reuter • Aug. 25, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Sponsored by PAXXUS
Sustainable medical device packaging solutions: Mono-materials that perform
Mono-material packaging meets tough healthcare demands while improving recyclability.
Aug. 25, 2025 -
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
By Nick Paul Taylor • Aug. 25, 2025 -
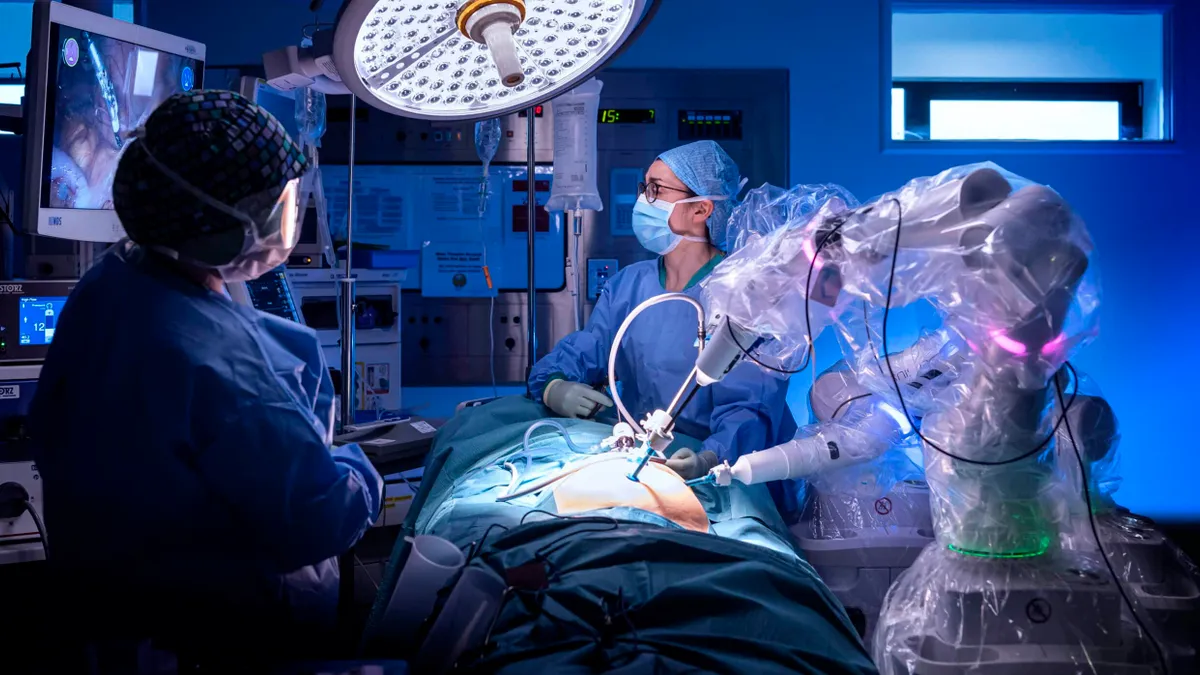
The robotic surgery market battle is heating up
After a busy summer of surgical robotics news, check out MedTech Dive’s roundup of coverage in the space.
By Susan Kelly • Aug. 22, 2025 -
MedTech Europe sounds alarm over impact of US-EU tariffs on patients
The trade group wants the U.S. to eliminate tariffs on medical technologies, warning that “patients cannot be collateral damage in trade tensions.”
By Nick Paul Taylor • Aug. 22, 2025 -
More than 1,100 devices have received the FDA’s breakthrough designation
The FDA updated its list of breakthrough devices as medtech groups lobby for faster Medicare coverage of products with the designation.
By Elise Reuter • Aug. 21, 2025 -
US, EU agree to terms of framework trade pact
The two trading partners documented provisions of a deal that would set a 15% tariff on many EU imports, including cars, auto parts, pharmaceuticals and semiconductors.
By Philip Neuffer • Aug. 21, 2025 -
AdvaMed urges Dr. Oz to speed Medicare coverage of breakthrough devices
The medtech trade group wants the CMS to take “bold action” to cut the lag between FDA authorization of devices and Medicare coverage.
By Nick Paul Taylor • Aug. 21, 2025 -
Q&A
How Aktiia built the first over-the-counter cuffless blood pressure monitor
Aktiia received FDA clearance in July for the first cuffless blood pressure monitor authorized to be sold over the counter.
By Elise Reuter • Aug. 20, 2025 -
NeuroOne gains FDA clearance for trigeminal nerve ablation
The minimally invasive system uses radiofrequency energy to target nerve tissue causing facial pain.
By Susan Kelly • Aug. 20, 2025 -
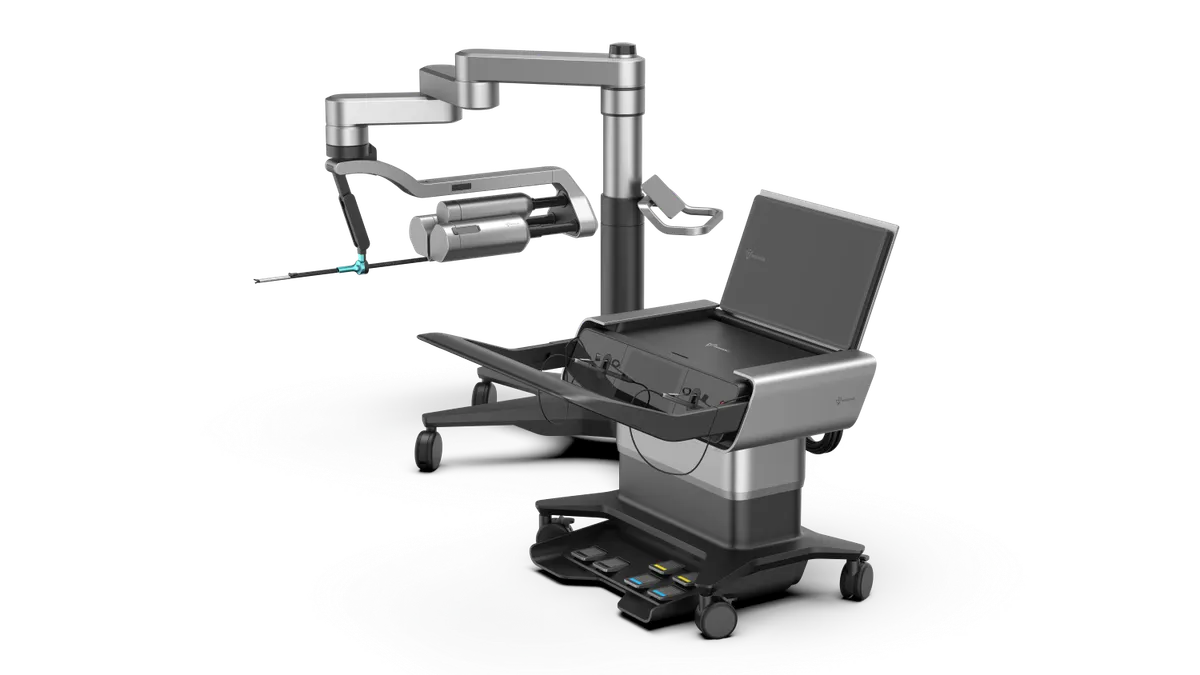
CMR Surgical hires Chris O’Hara to drive US robot expansion
O’Hara joins CMR’s executive team as commercial president and general manager for the U.S., following the FDA’s authorization last fall for its Versius system.
By Susan Kelly • Aug. 19, 2025 -
Medtronic recall of heart vent catheters tied to 3 serious injuries
The FDA said the request to quarantine the devices followed reports of the products “resisting shape retention when being bent.”
By Nick Paul Taylor • Aug. 19, 2025 -
Heart devices pace medtech’s summer funding flows
July and August have brought a steady beat of private financings for young medtech companies, with several deals supporting new technologies to treat heart conditions.
By Susan Kelly • Aug. 18, 2025 -
Philips will spend $150M to expand manufacturing for AI-enabled tech
The investment includes increasing manufacturing and research and development capabilities at its Reedsville, Pennsylvania, facility.
By Elise Reuter • Aug. 15, 2025 -
Trump says semiconductor tariffs to come next week
The president indicated that duties on chips would be “approximately 100%” earlier this month.
By Philip Neuffer • Aug. 15, 2025 -
Apple updates smart watches with blood oxygen feature after customs ruling
Apple said it was able to bring a redesigned blood oxygen feature to its devices after a recent U.S. customs ruling.
By Elise Reuter • Aug. 14, 2025 -
Reprieve Cardiovascular raises $61M; Conformal Medical nets $32M for LAAO
The fresh funding will support pivotal trials in progress and commercialization preparations at the two heart-focused companies.
By Susan Kelly • Aug. 14, 2025 -
Vicarious Surgical delays robot timeline
Days into the job, CEO Stephen From said the company no longer expects to begin a clinical trial for its robotic system by the end of the year.
By Susan Kelly • Aug. 13, 2025 -
Q&A
Shan Jegatheeswaran shares J&J’s vision for AI in the OR
By combining surgical video with other insights, Jegatheeswaran hopes Johnson & Johnson can support better outcomes in procedures.
By Elise Reuter • Aug. 13, 2025 -
Cardiosense appoints John Martin as chief medical officer
The appointment comes weeks after the company received 510(k) clearance for its wearable heart monitor.
By Nick Paul Taylor • Aug. 13, 2025 -
Draeger removes ventilation filters over misleading carbon dioxide readings
Hospitals are being advised to stop using the filters after serious injuries were reported related to the problem, the FDA said in a recall notice.
By Elise Reuter • Aug. 12, 2025 -
Heartflow’s IPO raises $364M
The company’s stock rose in its first two days on public markets, closing on Monday up almost 58% over the IPO price amid investor enthusiasm.
By Nick Paul Taylor • Aug. 12, 2025 -
US to maintain lower tariff rates on China imports for 90 more days
China also extended its suspension of additional retaliatory duties against U.S. goods, maintaining current rates until November.
By Max Garland • Aug. 12, 2025 -
SetPoint Medical secures $140M to fund neuromodulation for arthritis
Abbott and Boston Scientific are among the investors in SetPoint, whose neurostimulation device gained FDA approval last month.
By Susan Kelly • Aug. 11, 2025