Medical Devices: Page 17
-
Abbott, Dexcom settle glucose monitor patent lawsuits
The rival companies agreed not to sue each other for the next 10 years.
By Elise Reuter • Jan. 2, 2025 -
Q&A
Few medical devices are designed for children. An FDA-Children’s National collaboration aims to change that.
Kolaleh Eskandanian, chief innovation officer at Children’s National Hospital, said the partnership is meant to address the many challenges with developing devices for children and infants.
By Elise Reuter • Jan. 2, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Top 10 medtech deals of 2024
Multibillion-dollar buyouts returned last year, led by Johnson & Johnson’s $13.1 billion acquisition of Shockwave Medical.
By Nick Paul Taylor • Jan. 2, 2025 -
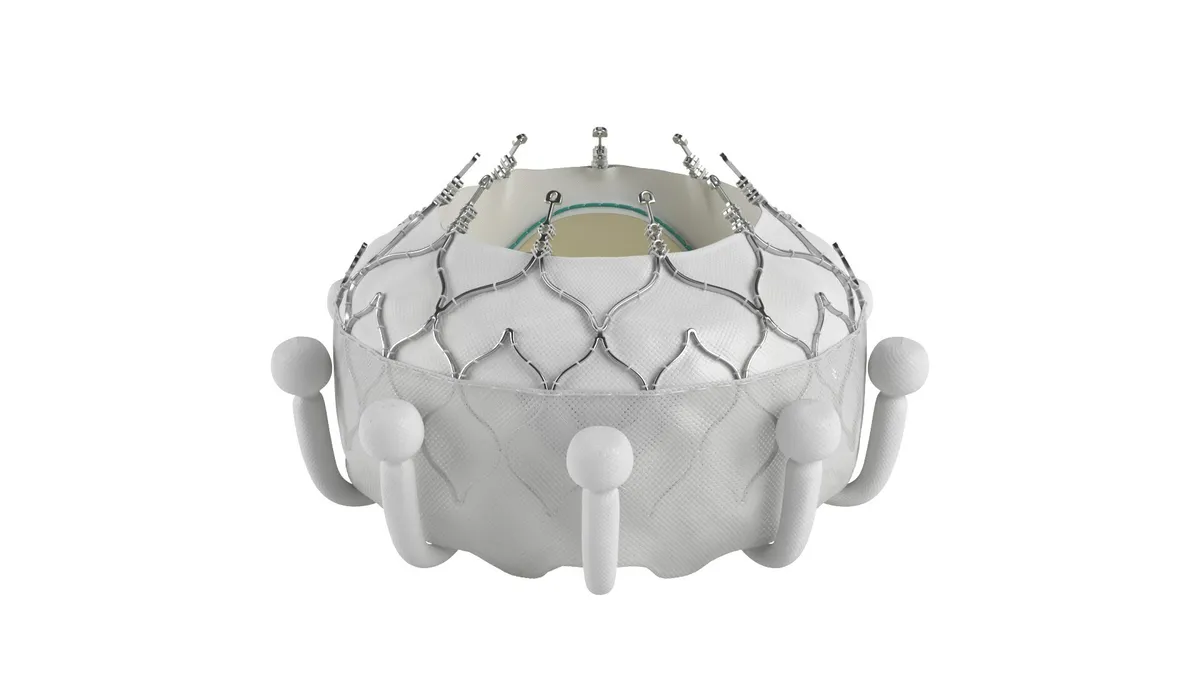
CMS proposes Medicare coverage for transcatheter tricuspid replacement
Edwards submitted a request for a national coverage determination in February, after receiving FDA approval for its Evoque device earlier that month.
By Elise Reuter • Updated Dec. 23, 2024 -
Robots, AI and PFA: The top medtech Q&As of 2024
From challenging market leaders to the growing influence of artificial intelligence, medical device executives had a lot to discuss this year. Check out MedTech Dive’s top Q&As of 2024.
By Ricky Zipp • Dec. 20, 2024 -
Heart valve developer Anteris launches IPO
IPOs in medtech have been sparse, but some analysts said improving market conditions could support a revival of offerings in 2025.
By Susan Kelly • Dec. 20, 2024 -
BD receives FDA warning letter over quality system violations
Inspectors found 111 open tickets for software defects categorized as catastrophic or severe patient harm. Several safety complaints were also listed that BD didn’t report within the required timeframe.
By Nick Paul Taylor • Dec. 20, 2024 -
Medical device recalls under the spotlight in 2024
Medtech regulators and watchdogs took a closer look at recalls in 2024. Here’s a recap of notable recalls and product safety actions in the past year.
By Elise Reuter • Dec. 19, 2024 -
Boston Scientific updates cryoablation catheter instructions after 4 death reports
The update followed a higher than expected number of reports of esophageal injury after catheter ablation procedures for atrial fibrillation.
By Nick Paul Taylor • Dec. 19, 2024 -

 Retrieved from Merit Medical on December 17, 2024
Retrieved from Merit Medical on December 17, 2024
Merit Medical president to resign after unspecified conduct allegations
Joseph Wright’s resignation is effective in January, and CEO and founder Fred Lampropoulos has been reappointed president.
By Susan Kelly • Dec. 18, 2024 -
Dexcom adds generative AI to over-the-counter CGMs
Dexcom this week began rolling out the feature, which analyzes data to provide personalized tips and recommendations.
By Elise Reuter • Dec. 18, 2024 -
Trump’s return to office brings uncertainty for medtech industry
With about one month before inauguration day, check out a roundup of MedTech Dive’s coverage on what President-elect Donald Trump’s new administration means for medical device companies.
By Ricky Zipp • Dec. 18, 2024 -
Medtech firms have cut thousands of jobs this year. Will layoffs continue in 2025?
Industry experts said profit pressures and M&A contributed to job cuts. They said layoffs will likely continue into next year, but some expect the cuts to slow.
By Elise Reuter • Dec. 18, 2024 -
Boston Scientific pacemaker recall tied to 832 injuries, 2 deaths
Certain Accolade pacemakers can permanently enter safety mode, which limits the devices’ ability to treat patients properly and requires early replacement.
By Ricky Zipp • Updated Feb. 25, 2025 -
BD to pay $175M to settle charges of misleading investors on Alaris pump
BD will pay a civil penalty to resolve charges it misled investors about risks associated with sales of its Alaris infusion pump, the Securities and Exchange Commission said.
By Elise Reuter • Dec. 17, 2024 -
Zimmer receives FDA nod for stemless shoulder implant
The Osseofit devices are intended to match patients’ shoulder bone anatomy and preserve healthy bone in total shoulder replacement procedures.
By Elise Reuter • Dec. 16, 2024 -
PFA to surpass radiofrequency ablation in 2025: Citi survey
Pulsed field ablation could soon be used in the majority of electrophysiology procedures to treat atrial fibrillation as Medtronic, Boston Scientific and Johnson & Johnson battle for market share.
By Susan Kelly • Dec. 16, 2024 -
FDA issues first early alert with Fresenius Kabi infusion pump recall
Fresenius Kabi is asking hospitals to remove certain pumps because of the risk of an alarm that could interrupt treatment.
By Elise Reuter • Dec. 13, 2024 -
Augmedics names Paul Ziegler as CEO
Augmedics sells an augmented reality headset that allows spine surgeons to view virtual images of the patient’s bones and surgical instruments inside the body. Ziegler is the former CEO of Viewray.
By Nick Paul Taylor • Dec. 13, 2024 -
Endoquest robotic system gets FDA OK to launch clinical trial
A pivotal study of the robotic platform in colorectal surgery will begin at five hospitals in early 2025.
By Susan Kelly • Dec. 13, 2024 -
Capstan Medical raises $110M for heart valve with robotic system
The startup’s third round of financing includes support from Intuitive Surgical’s venture capital arm.
By Susan Kelly • Dec. 12, 2024 -
4 takeaways from the FDA’s first digital health advisory committee
Industry and patient representatives debated how the FDA should regulate generative AI in medical devices and address new challenges with the technology, such as the use of complex models that can change quickly.
By Elise Reuter • Dec. 12, 2024 -
Masimo lays off 75 employees in California
Under new leadership, the patient monitoring company is allocating resources to fewer projects as it focuses on market opportunities that address unmet needs, a spokesperson said.
By Susan Kelly • Dec. 11, 2024 -
Patterson, a dental products distributor, agrees to $4.1B sale to healthcare investor
Last week, Patterson Companies announced it would seek strategic alternatives after the company’s profit decreased by one-third in its fiscal second quarter.
By Elise Reuter • Dec. 11, 2024 -
How Trump’s second term will affect the medtech industry
From M&A and tariffs to new health leaders, here are the top issues for the medtech industry to watch during President Donald Trump’s return to office.
By Ricky Zipp • Dec. 10, 2024