Medical Devices: Page 25
-
Embecta gets FDA nod for insulin patch pump
Embecta’s first patch pump features a larger insulin reservoir, based on feedback from people with Type 2 diabetes.
By Elise Reuter • Sept. 3, 2024 -
Illumina avoids fine for Grail purchase in European court victory
Illumina will avoid a penalty of 432 million euros after a court ruled the European Commission did not have jurisdiction to challenge the company’s Grail acquisition.
By Susan Kelly • Sept. 3, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtronic’s chief medical officer of acute care and monitoring departs for new role
Sam Ajizian leaves Medtronic six months after the company reversed a plan to split off its patient monitoring business.
By Nick Paul Taylor • Aug. 30, 2024 -
FDA finalizes Voluntary Malfunction Summary Reporting guidance
The agency clarified when companies can submit device malfunction reports in a quarterly summary, instead of individual reports every 30 days.
By Nick Paul Taylor • Aug. 30, 2024 -
Penumbra axes virtual reality division, will lay off 71 people
CEO Adam Elsesser told investors the company’s focus needs to be on its core thrombectomy business as it cut its sales forecast for 2024.
By Elise Reuter • Aug. 29, 2024 -
Johns Hopkins, CareFirst, Techstars launch healthcare AI accelerator
The 13-week program will offer funding and guidance to up to 12 health tech, medtech and biotech startups working on AI tools.
By Emily Olsen • Aug. 29, 2024 -

 Retrieved from UFP Technologies on August 27, 2024
Retrieved from UFP Technologies on August 27, 2024
UFP to buy device component maker AQF Medical, extending deal spree
AQF makes foam and thermoplastic components for use in medical devices and packaging.
By Nick Paul Taylor • Aug. 28, 2024 -
Boston Scientific receives CE mark for updated TAVR technology
Changes include the addition of a larger valve size that company executives have said will open up 25% of the market in Europe.
By Nick Paul Taylor • Aug. 28, 2024 -
Q&A
Insulet’s Trang Ly on overcoming ‘clinical inertia’ in diabetes care
Automated insulin delivery for Type 2 patients can help change minds among physicians who have been reluctant to prescribe pump therapy, the chief medical officer said in an interview.
By Susan Kelly • Aug. 27, 2024 -
Inari updates label of clot removal device linked to 6 deaths
Inari recalled the catheter and revised instructions for use after reports of the device becoming entrapped or blocking patients’ lung arteries.
By Nick Paul Taylor • Aug. 27, 2024 -
Siemens Healthineers to grow PET imaging with purchase of Novartis assets
Siemens will pay 200 million euros for the manufacturing and distribution network of Novartis’ radiopharmaceutical molecular imaging business.
By Nick Paul Taylor • Aug. 27, 2024 -
Insulet expands Omnipod 5 pump to people with Type 2 diabetes
The FDA clearance marks the first automated insulin delivery system for people with Type 2 diabetes.
By Elise Reuter • Aug. 26, 2024 -
Dexcom prices first over-the-counter glucose monitor
Known as Stelo, the device is intended for people who don’t take insulin and costs $89 per month.
By Elise Reuter • Aug. 26, 2024 -
Defibtech recalls chest compression device linked to patient death
Customers are asked to return affected devices due to a problem in the motor that could stop compressions.
By Nick Paul Taylor • Aug. 26, 2024 -
FDA seeks feedback on predetermined change control plans
The guidance describes changes companies can make to medical devices without filing a new 510(k) or premarket approval supplement.
By Nick Paul Taylor • Aug. 26, 2024 -

 Retrieved from Stryker on August 23, 2024
Retrieved from Stryker on August 23, 2024
Stryker to add back pain treatment with Vertos Medical buy
Stryker’s latest purchase comes amid a pickup in medtech M&A, with Johnson & Johnson, Boston Scientific and Edwards Lifesciences all active this year.
By Susan Kelly • Aug. 23, 2024 -
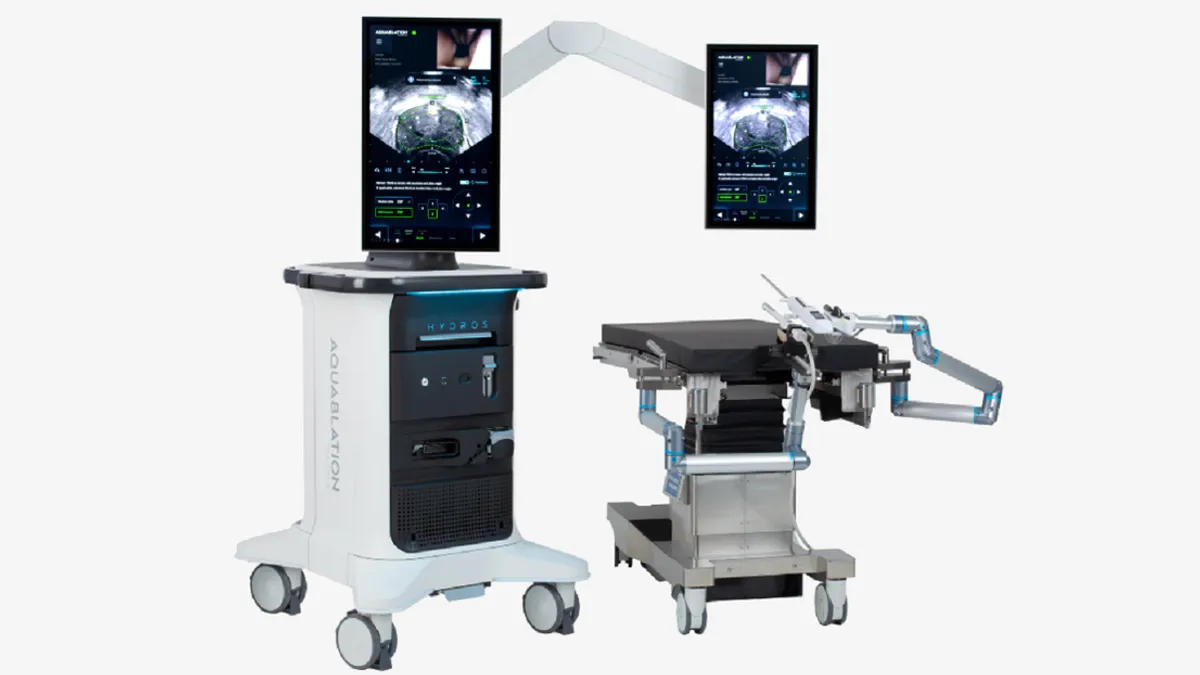
Procept wins FDA clearance for updated prostate surgery robot
Procept Biorobotics has added AI capabilities and switched to a single-use scope to support mass-market adoption.
By Nick Paul Taylor • Aug. 22, 2024 -
Zoll to buy Vyaire’s ventilator business with $37M auction bid
Vyaire Medical held a virtual auction of assets after filing for Chapter 11 bankruptcy protection in June.
By Nick Paul Taylor • Aug. 21, 2024 -

 Retrieved from Masimo on August 20, 2024
Retrieved from Masimo on August 20, 2024
Masimo’s consumer audio brands attract more suitors
Negotiations with a potential joint venture partner will continue while Masimo initiates discussions with new parties that have expressed interest in the consumer audio business.
By Susan Kelly • Aug. 21, 2024 -

 "Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
"Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
FDA’s plan to expand device surveillance faces challenges, GAO finds
Funding issues and limited use of unique device identifiers are hurdles to expanding an FDA system to monitor medical device safety, the federal watchdog said.
By Nick Paul Taylor • Aug. 21, 2024 -
J&J to buy heart failure implant maker V-Wave for up to $1.7B
J&J has agreed to pay $600 million upfront for the private company as it works to expand its portfolio of cardiovascular devices.
By Susan Kelly • Aug. 20, 2024 -
Edwards continues M&A spree with Genesis’ TAVR tech
Edwards, which has made a series of acquisitions in recent months, is also investing $25 million in Singapore-based Genesis Medtech.
By Susan Kelly • Aug. 19, 2024 -
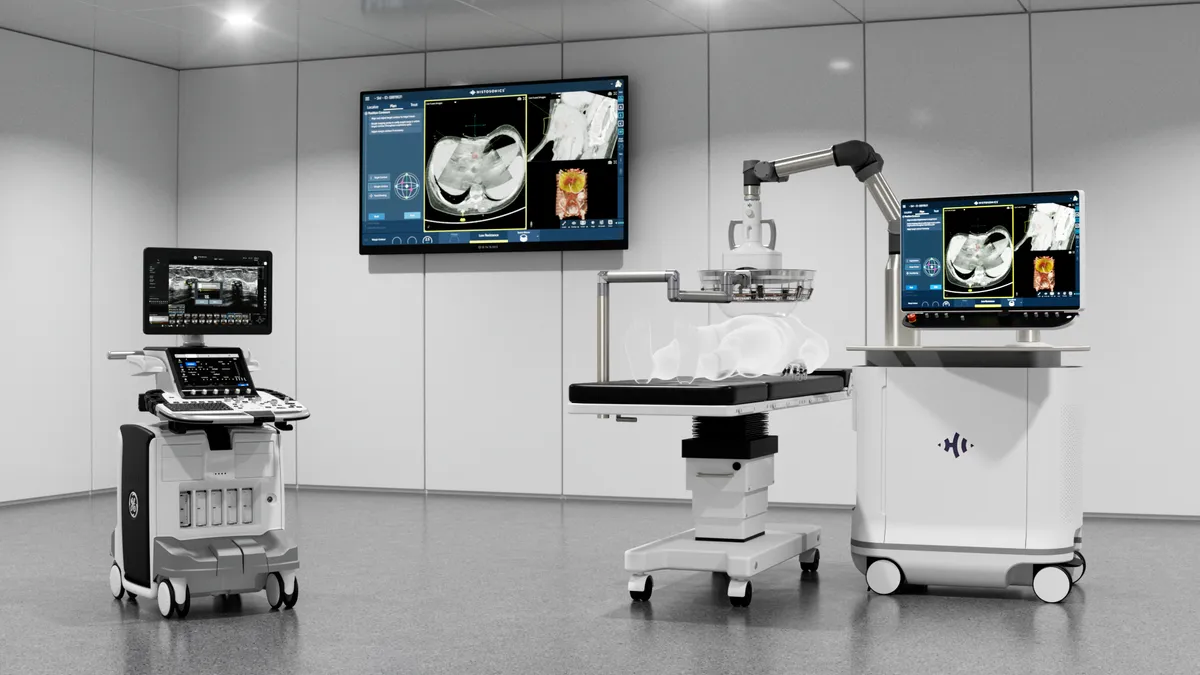
Histosonics raises $102M for noninvasive treatment to destroy tumors
Using sound waves to break down tumor tissue can offer patients with liver cancer an alternative to surgery, radiation and chemotherapy.
By Susan Kelly • Aug. 19, 2024 -

 Erin Scott / U.S. White House. Retrieved from Flickr.
Erin Scott / U.S. White House. Retrieved from Flickr.
Biden pledges $150M for medical tech to improve cancer surgery
Awards include funding for microscopy and imaging tools to help surgeons ensure all cancer is removed during a procedure.
By Elise Reuter • Aug. 16, 2024 -
Stereotaxis earns CE mark for new surgical robot
CEO David Fischel said the platform, called GenesisX, is designed to address challenges hospitals have faced when installing the company’s earlier robotic systems.
By Susan Kelly • Aug. 15, 2024