Medical Devices: Page 36
-
US hospitals report increased procedure volumes, spending outlook in Q4 survey
Truist analysts wrote that Medtronic, Boston Scientific and Edwards Lifesciences could benefit from rising cardiology volumes, while Intuitive Surgical may benefit from improved capital spending.
By Nick Paul Taylor • Jan. 8, 2024 -
Nanowear gets FDA clearance for undergarment that estimates blood pressure
The company’s continuous blood pressure monitor allows clinical investigators to remotely capture data on patients.
By Nick Paul Taylor • Jan. 5, 2024 -
Thermo Fisher to lay off 74 California workers in restructuring
The scientific instruments maker, which has been streamlining operations to cut costs, said the latest job reductions would be effective Feb. 1.
By Susan Kelly • Jan. 5, 2024 -
Renovos secures FDA breakthrough status for bone graft alternative
When injected, Renovite acts as a scaffold for cells and helps localize and retain molecules that stimulate healing.
By Nick Paul Taylor • Jan. 5, 2024 -
FDA warns providers not to buy or implant Synovo’s total hip system
Last year, the FDA sent a warning letter to Synovo after finding that its femoral resurfacing cup had been “significantly changed or modified.”
By Nick Paul Taylor • Jan. 4, 2024 -
Apple Watch sales resume after latest twist in Masimo patent case
An appeals court has granted Apple’s emergency motion to pause the U.S. International Trade Commission’s ban on sales of its watches with a pulse oximetry feature.
By Susan Kelly • Jan. 3, 2024 -
Boston Scientific pulls forward expected approval for Farapulse PFA system
Analysts said a first quarter approval would increase their confidence in the company’s potential for sales growth of 10% or more.
By Nick Paul Taylor • Jan. 3, 2024 -
Roche to buy LumiraDx’s point-of-care technology for $295M
LumiraDx faces financial pressures amid declining test sales and a potential delisting from the Nasdaq.
By Elise Reuter • Jan. 2, 2024 -
Philips recalls MRI machines due to risk of explosion
The Food and Drug Administration labeled the recall as a Class I event. There has been one report of a machine exploding in the 22 years the system has been in use.
By Nick Paul Taylor • Dec. 22, 2023 -
Agilent to lay off about 400 workers amid site closures
Most of the reductions, which equal about 2% of the company’s global workforce, will be completed in the first quarter, Agilent said.
By Susan Kelly • Dec. 22, 2023 -
Resmed posts notice about risk of mask magnets interfering with medical implants
Like its rival Philips, Resmed has determined that patients should not wear magnetized masks near some implants.
By Nick Paul Taylor • Dec. 22, 2023 -
SEC charges former Stimwave CEO with $41M fraud scheme
The Securities and Exchange Commission alleges that Laura Tyler Perryman approved the creation of “fake” implantable parts for the company’s PNS device and defrauded investors of $41 million.
By Ricky Zipp • Dec. 21, 2023 -
Apple to pause US sales of smartwatches with pulse oximetry
The tech giant’s move follows a USITC ruling that found the watches infringed some of Masimo's patents for measuring blood oxygen.
By Susan Kelly • Dec. 20, 2023 -
FDA drafts guidance outlining real-world evidence for medical device submissions
By issuing the new guidance, the agency is meeting the requirements of a year-end spending bill.
By Elise Reuter • Dec. 19, 2023 -
Zimvie to sell spine business for $375M
Zimvie said the sale to H.I.G. Capital will leave the company focused on its dental business and help reduce debt.
By Elise Reuter • Dec. 18, 2023 -
BTIG analysts optimistic medtech will rebound in 2024 after ‘string of rough years’
Rising interest rates, fears about the impact of GLP-1 drugs and “choppy supply-chain dynamics” dragged on the industry this year, the analysts wrote.
By Nick Paul Taylor • Dec. 18, 2023 -
Glaukos wins FDA approval for drug-releasing eye implant to treat glaucoma
William Blair analysts said the product “should revolutionize how glaucoma is treated by addressing noncompliance with drops.”
By Nick Paul Taylor • Dec. 15, 2023 -
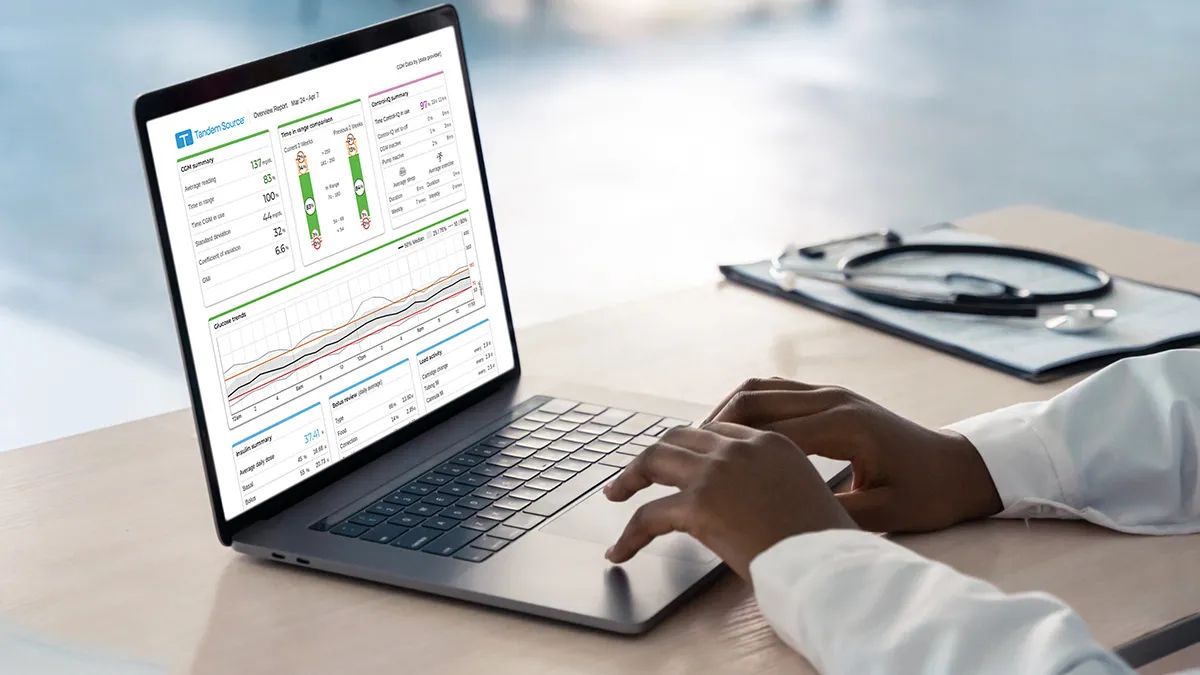
Tandem completes US launch of revamped diabetes management platform
The platform, Tandem Source, combines the features of the company’s legacy t:connect offerings with new data reports.
By Nick Paul Taylor • Dec. 15, 2023 -
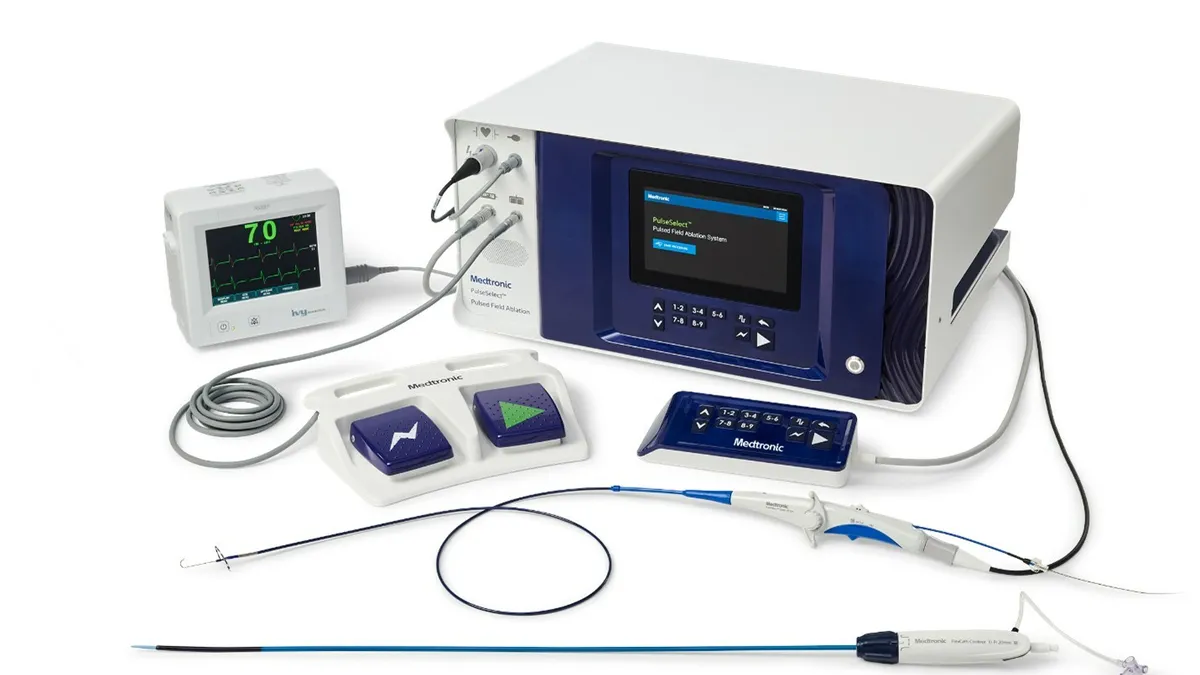
Medtronic snags first pulsed field ablation approval in US
The treatment for atrial fibrillation has gained attention as a safer alternative to radiofrequency and cryoablation. Boston Scientific and Johnson & Johnson are also pursuing the PFA market.
By Susan Kelly • Dec. 14, 2023 -
Resmed hails ‘significant victory’ as patent board rules in dispute with NYU
The decisions invalidate claims that NYU argued were infringed by Resmed’s sleep apnea devices.
By Nick Paul Taylor • Dec. 14, 2023 -
In wake of Philips recall, senators urge review of FDA medical device oversight
Sens. Richard Durbin and Richard Blumenthal said the sleep apnea device maker “did nothing” while patients suffered.
By Susan Kelly • Dec. 14, 2023 -
Integra to buy J&J’s Acclarent for $275M
Integra said the purchase, which includes a portfolio of balloon dilation products for the sinuses and eustachian tube, would make it a market leader in ENT procedures.
By Elise Reuter • Dec. 13, 2023 -
Zimvie’s collaboration with Brainlab delivers 510(k) win for spinal fixation system
Amid recent struggles in its spine unit, Zimvie has been expanding its Brainlab partnership.
By Nick Paul Taylor • Dec. 13, 2023 -
The FDA approved 2 renal denervation devices. There are still questions about who will benefit.
The devices from Recor and Medtronic are intended to treat high blood pressure. An advisory panel backed the former, but recommended against approval of the latter.
By Elise Reuter • Dec. 13, 2023 -
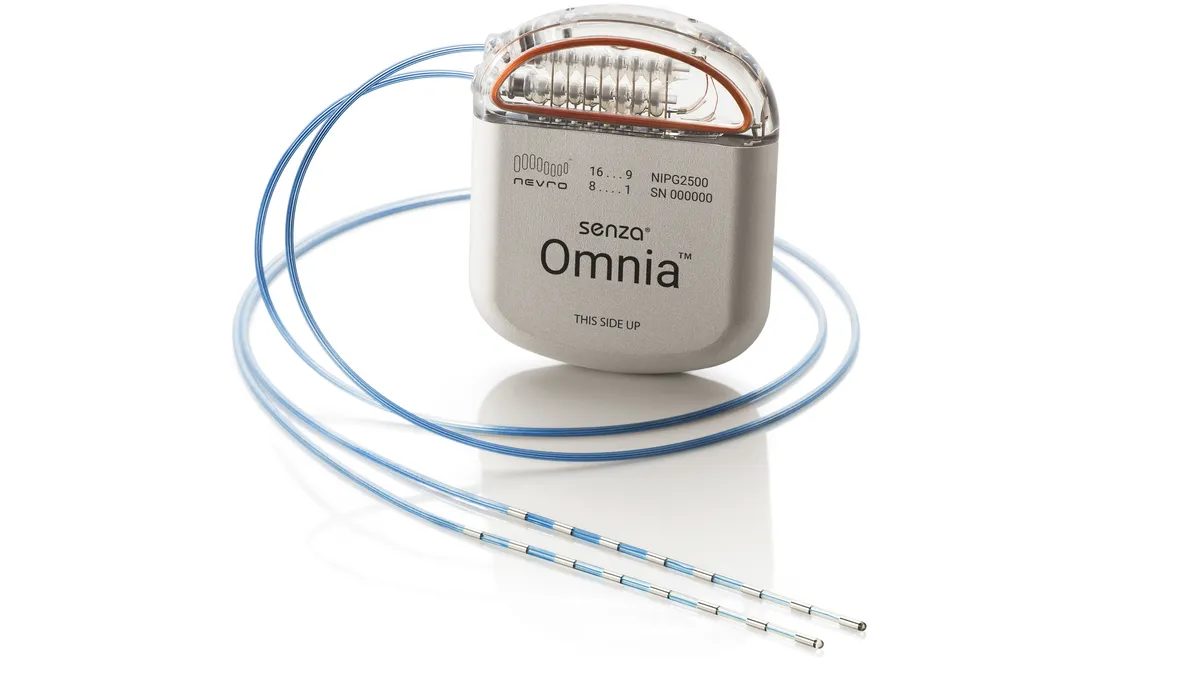
Activist investor takes stake in Nevro amid struggles to grow neuromodulation sales
Engaged Capital is opposed to Nevro going after acquisitions that may jeopardize its core business, Bloomberg reported Monday.
By Nick Paul Taylor • Dec. 12, 2023