Medical Devices: Page 38
-
‘Fire, smoke, burns’: FDA warns about new safety risks for Philips CPAP machines
Philips recently filed more than 270 reports of thermal problems related to its DreamStation 2 device from the past three years.
By Elise Reuter • Nov. 28, 2023 -

 Retrieved from Orthofix on September 12, 2023
Retrieved from Orthofix on September 12, 2023
Orthofix hires new CEO, 2 months after former leaders ousted
Massimo Calafiore is expected to join the spine and orthopedic device maker in early 2024 from Italy-based LimaCorporate.
By Susan Kelly • Nov. 28, 2023 -
Unomedical recall of infusion sets tagged as Class I by FDA
The company notified Tandem Diabetes Care, its sole consignee, in October of the risk for infusion sets to detach from insulin pumps, disrupting insulin delivery.
By Nick Paul Taylor • Nov. 28, 2023 -
Medtronic to chase Boston Scientific, Abbott in left atrial appendage closure market
The medtech giant acquired the Penditure LAA exclusion system in August from Miami-based medical device incubator Syntheon.
By Susan Kelly • Updated Dec. 6, 2023 -
Roundup: Top medtech companies cut jobs in 2023
Johnson & Johnson, Medtronic and Abbott were among the top companies to announce layoffs this year as the industry implemented cost-cutting measures.
By Ricky Zipp • Nov. 27, 2023 -
Deep Dive
Why medical device companies are worried about the EPA’s planned sterilization regs
The EPA is expected to finalize new regulations in March that would limit ethylene oxide emissions from companies that sterilize medical devices.
By Elise Reuter , Shaun Lucas • Nov. 27, 2023 -
FDA withdraws from Global Harmonization Working Party
The agency has become “concerned with the divergent harmonization efforts for medical devices” and will focus on working with the International Medical Device Regulators Forum going forward.
By Elise Reuter • Nov. 27, 2023 -
LivaNova warns cybersecurity incident disrupts systems, takes steps to limit impact
The medical device maker warned that the incident is expected to continue to disrupt its business operations.
By Nick Paul Taylor • Nov. 27, 2023 -
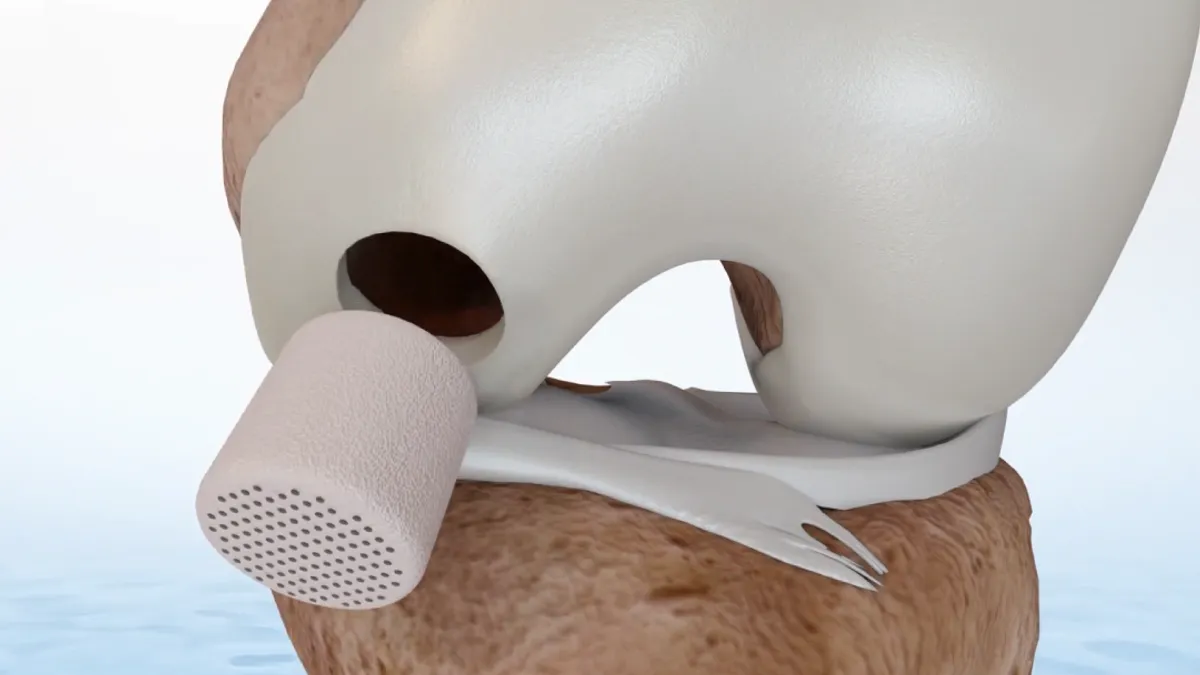
Smith & Nephew inks $180M CartiHeal buyout, capitalizing on Bioventus turmoil to land knee implant
Bioventus’ protracted buyout bid ultimately fell apart and opened the door to Smith & Nephew.
By Nick Paul Taylor • Nov. 27, 2023 -
FDA weighs in on SoClean’s field correction for CPAP cleaning machines
SoClean announced a voluntary field action last week to address user complaints related to improper set-up and unauthorized modifications.
By Nick Paul Taylor • Nov. 22, 2023 -
Medtronic wins pulsed field ablation CE mark, teeing up 2-front challenge to Boston Scientific
Medtronic received the mark for its single-shot PFA device, clearing the company to expand its portfolio of European atrial fibrillation devices.
By Nick Paul Taylor • Nov. 22, 2023 -
BD names ex-3M exec Ronald Silverman as chief medical officer
Silverman left 3M three months after the company installed a new leader at its health business.
By Nick Paul Taylor • Nov. 22, 2023 -
Medtronic CEO downplays the impact of obesity drugs on bariatric surgeries, diabetes unit
The company is the latest medtech to address concerns about how weight loss drugs may impact growth.
By Ricky Zipp • Nov. 21, 2023 -
FDA concerned Cardinal Health failed to mitigate risk of incompatible syringes
Days after posting the Class 1 recall notice, the FDA said changes made to certain syringes could lead to overdose, underdose, or delays in therapy or alarms.
By Nick Paul Taylor • Nov. 21, 2023 -
Medtronic receives FDA approval for renal denervation device
The company received approval for the hypertension treatment despite a mixed vote from an FDA advisory panel.
By Elise Reuter • Nov. 20, 2023 -
B. Braun infusion pump safety correction linked to 51 complaints, one death
While no devices need to be removed, the FDA labeled the correction a Class I recall due to the risk of injury or death for patients.
By Nick Paul Taylor • Nov. 20, 2023 -
Q3 recap: Medtech firms grapple with layoffs, GLP-1s and China slowdown
Despite these challenges, companies also flagged improvements in supply chain dynamics and pricing that could boost their earnings.
By Elise Reuter • Updated Nov. 21, 2023 -
FDA acts on industry call for clarity about scope of device shortage reporting rules
The agency posted a final guidance and draft guidance on medical device shortage reporting requirements, addressing industry concerns.
By Nick Paul Taylor • Nov. 17, 2023 -
FDA seeks feedback on racial bias of pulse oximeters, convenes advisory committee
The agency published a paper on improving the evaluation of pulse oximeters to take skin pigmentation into account.
By Nick Paul Taylor • Nov. 17, 2023 -
LetsGetChecked secures first FDA authorization for at-home chlamydia and gonorrhea test
Jeff Shuren, director of the Center for Devices and Radiological Health, said the test will give patients “more information about their health from the privacy of their own home.”
By Nick Paul Taylor • Nov. 16, 2023 -
FDA-ordered report tackles how to manage cybersecurity risks of legacy devices
The report from MITRE proposes multiple actions for protecting older medical devices, including research into more modular devices and collecting data on cyber risks.
By Nick Paul Taylor • Nov. 16, 2023 -
Vicarious delays surgical robot as integration challenges, R&D cuts slow development
Analysts at BTIG warned the company is “walking a very tight rope” and downgraded the stock.
By Nick Paul Taylor • Updated Nov. 15, 2023 -
 Retrieved from Food and Drug Administration.
Retrieved from Food and Drug Administration.
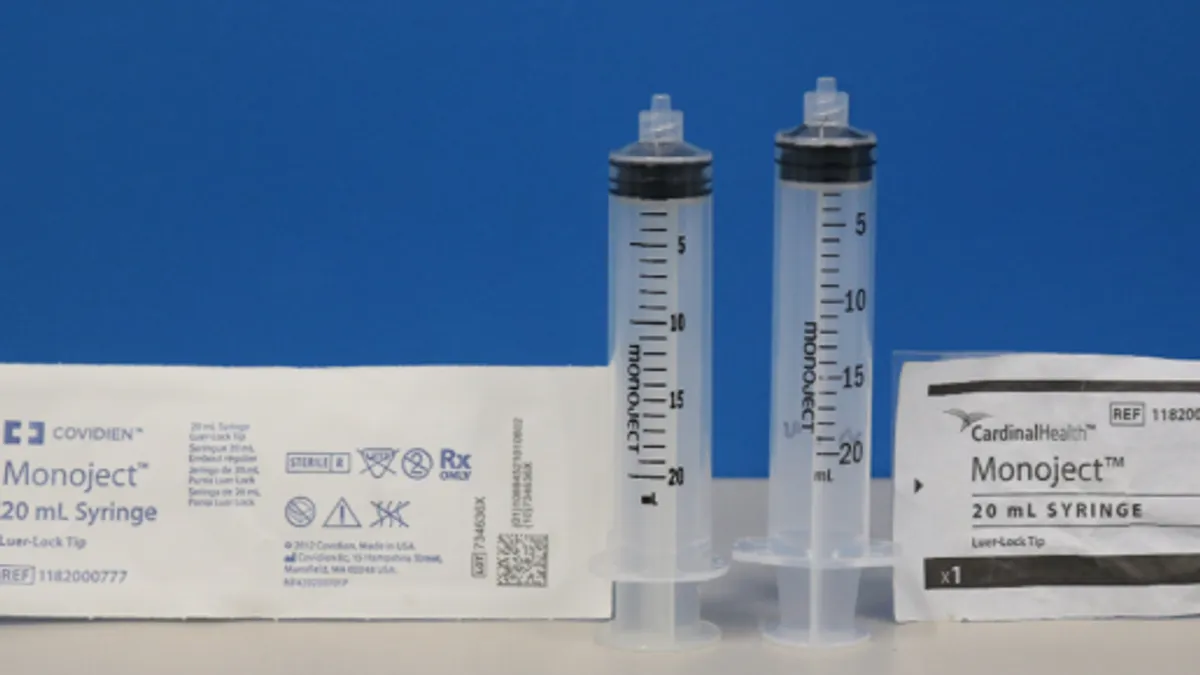
Cardinal’s changes to disposable syringes trigger FDA Class I recall notice
The problem is linked to 15 reports of delayed therapy and 13 reports of inaccurate dispensing.
By Nick Paul Taylor • Nov. 15, 2023 -

 Retrieved from Medtronic on October 05, 2023
Retrieved from Medtronic on October 05, 2023
Stolen Medtronic laryngoscopes deemed Class 1 recall
Some of the defective devices turned up on Facebook Marketplace before the posting was taken down, the FDA said in the recall notice.
By Susan Kelly • Nov. 15, 2023 -
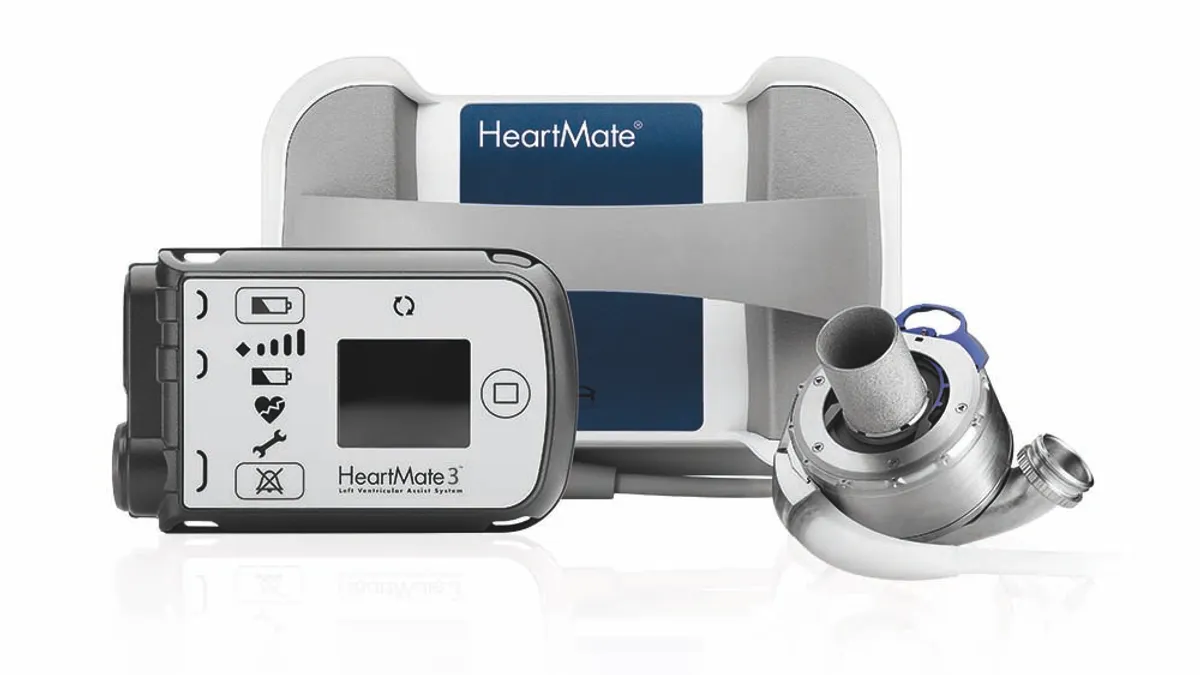
Abbott study links aspirin-free regimen to better outcomes in heart pump patients
An accompanying editorial said the “data provide an opportunity to immediately improve the outcomes of patients implanted with contemporary LVADs.”
By Nick Paul Taylor • Nov. 15, 2023