Medical Devices: Page 41
-
Abbott’s resorbable scaffold improves outcomes in below-the-knee artery disease, study finds
The device maker plans to submit the Esprit BTK data to the Food and Drug Administration for review.
By Nick Paul Taylor • Oct. 26, 2023 -
Boston Scientific study results support coronary drug-coated balloon in US
The results, shared at TCT 2023, found the company’s drug-coated balloon performed better than an uncoated balloon in procedures to reopen blocked arteries.
By Elise Reuter • Oct. 25, 2023 -
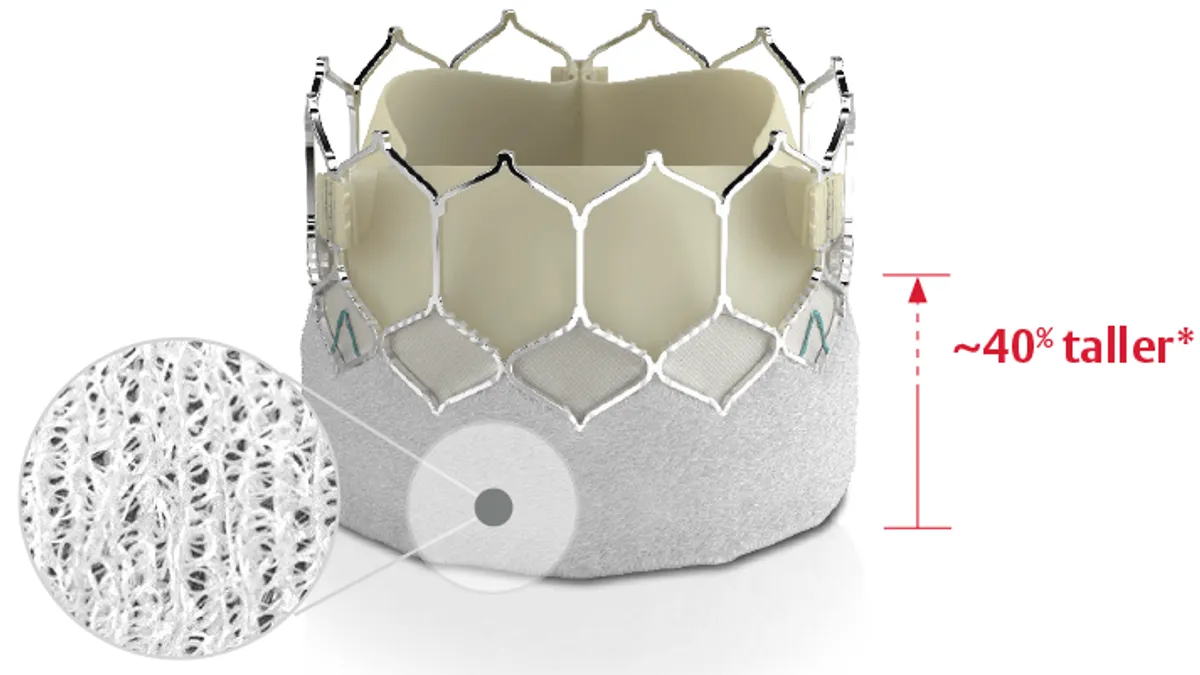
Edwards, Medtronic post low-risk TAVR data, allaying fears of market disruption
The late-breaking results, shared at TCT 2023, will have little impact on clinical practice or market share, J.P. Morgan and Stifel analysts predicted.
By Nick Paul Taylor • Oct. 25, 2023 -
Thermo Fisher cuts 2023 forecast, citing volatile economic conditions
Customer spending remains constrained overall, while economic conditions in China have weakened, CEO Marc Casper said on a Wednesday earnings call.
By Susan Kelly • Oct. 25, 2023 -
GE HealthCare, Novo Nordisk partner to use ultrasound to treat diabetes, obesity
The collaborators will study the use of peripheral focused ultrasound as a non-invasive, non-pharmacological method of managing blood glucose.
By Nick Paul Taylor • Oct. 25, 2023 -
Fresenius’ work to replace hemodialysis tubing flagged as Class I recall by FDA
Agency officials warned healthcare professionals last year about the potential for silicone tubing to expose patients to toxic compounds.
By Nick Paul Taylor • Oct. 25, 2023 -
5 takeaways from the FDA’s updated list of cleared AI/ML medical devices
The agency recently added 171 new devices to its list, with radiology once again accounting for the most authorizations.
By Elise Reuter • Oct. 24, 2023 -
J&J MedTech Chairman Ashley McEvoy to step down
McEvoy is leaving the company after 27 years to pursue other opportunities. Tim Schmid will now lead the MedTech business.
By Elise Reuter • Updated Oct. 24, 2023 -
Olympus recall of abdominal insufflation devices linked to reports of 10 serious injuries, 1 death: FDA
The Food and Drug Administration labeled the recall a Class I event.
By Ricky Zipp • Updated Oct. 30, 2023 -
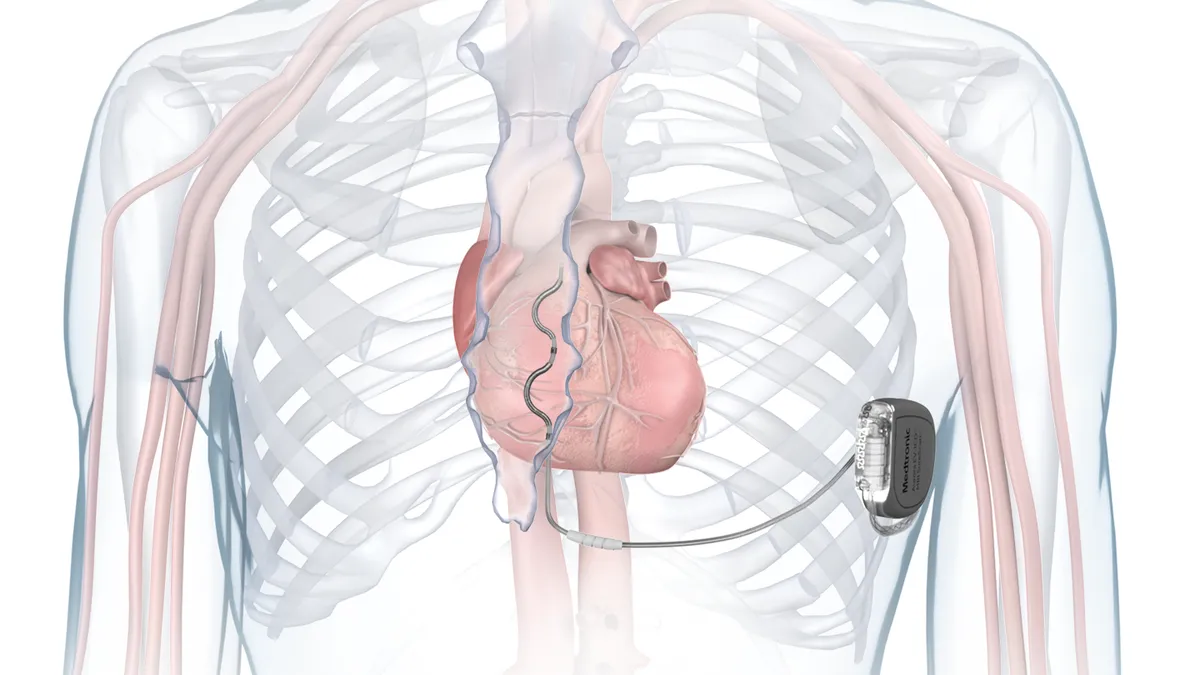
Medtronic gains FDA approval for extravascular defibrillator
The device to treat abnormal heart rhythms is designed to avoid risks associated with traditional implantable cardioverter defibrillators that have leads running through the veins to the heart.
By Susan Kelly • Oct. 23, 2023 -
Insulet receives FDA clearance for Omnipod 5 iPhone app
The application removes the need for patients with iPhones to carry a separate controller to manage bolus dosing.
By Elise Reuter • Oct. 23, 2023 -
Philips’ orders fall in Q3, overshadowing sales growth and return to sleep apnea market
CEO Roy Jakobs said during a third-quarter earnings call that there is still “significant demand” for the company’s sleep apnea products.
By Nick Paul Taylor • Oct. 23, 2023 -
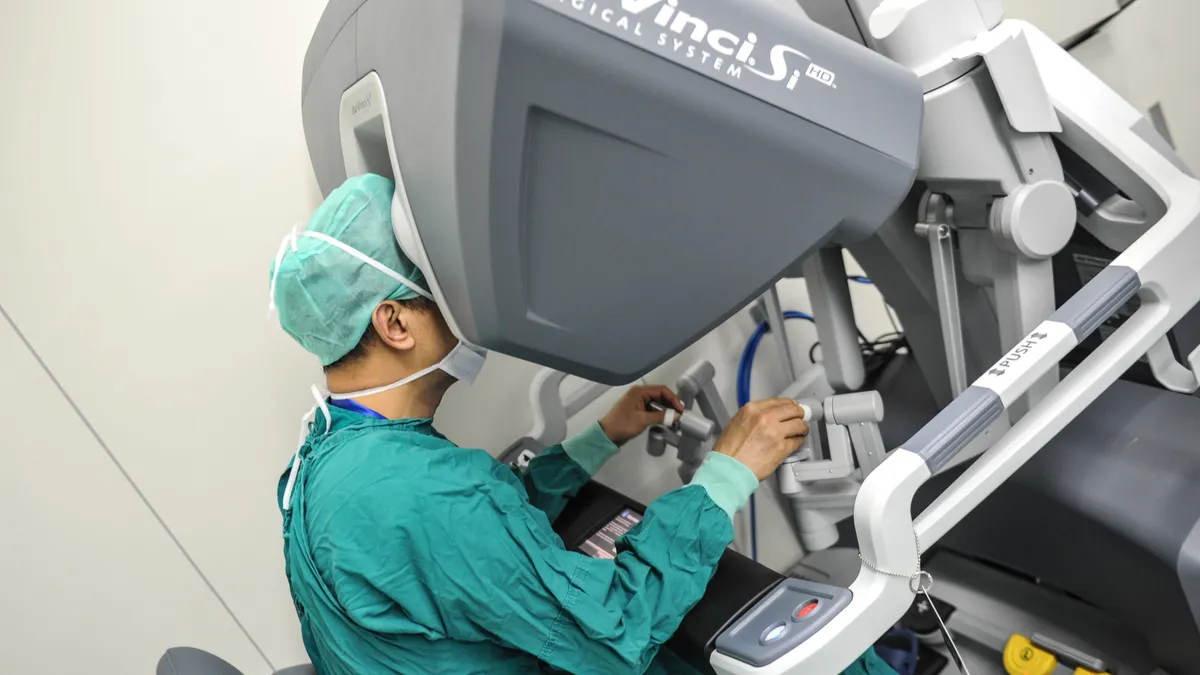
Intuitive’s Q3 sales miss estimates as robot leasing, China demand weigh
The robotic surgery leader’s overall procedure growth, however, exceeded market expectations.
By Susan Kelly • Oct. 20, 2023 -
AdvaMed CEO warns Congress of supply shortages from EtO, PFAS regulations
The trade group leader called for changes to the EPA’s proposals, while a scientist with UCSF said lawmakers should turn to science free of vested financial interests.
By Elise Reuter • Oct. 20, 2023 -
Physicians forecast Edwards’ 5-year data will have limited impact on low-risk TAVR market
Some doctors think TAVR may perform numerically worse but see little impact on the market unless there is a statistically significant result.
By Nick Paul Taylor • Oct. 20, 2023 -
Anumana names Boston Scientific veteran Maulik Nanavaty as CEO
Nanavaty has taken up the post after spending 18 years at Boston Scientific, where he held senior roles in interventional cardiology and neuromodulation.
By Nick Paul Taylor • Oct. 20, 2023 -
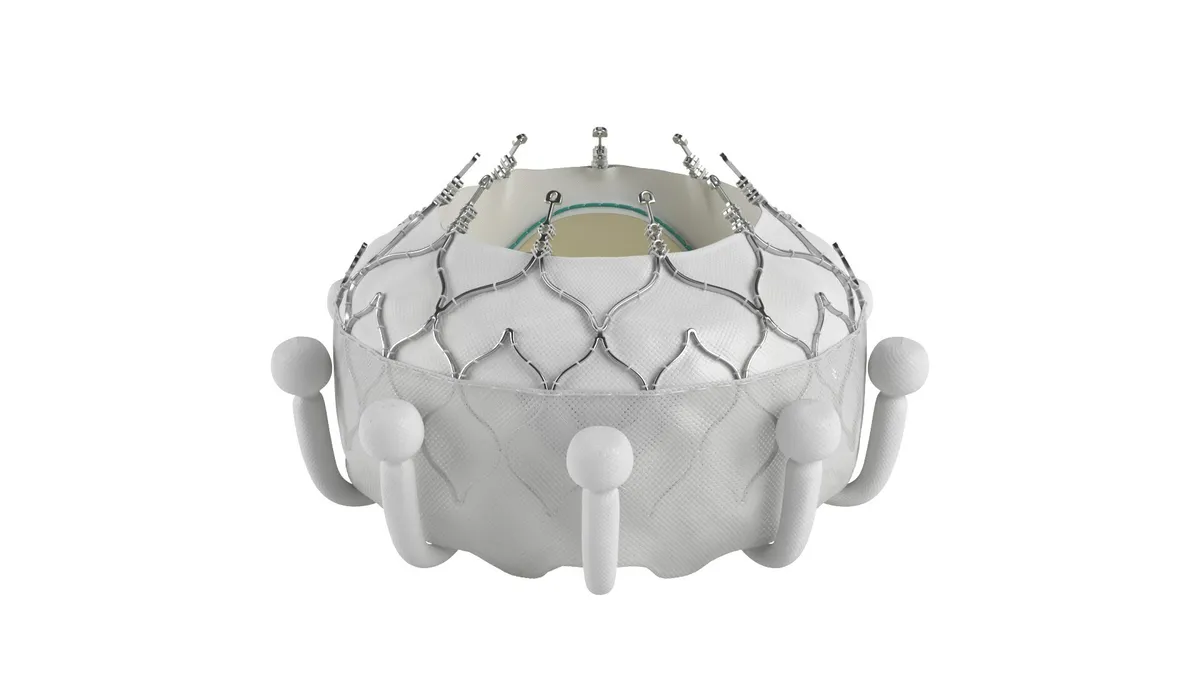
Edwards receives CE mark for tricuspid valve replacement
The company expects to gain U.S. approval late next year.
By Elise Reuter • Oct. 19, 2023 -
Supply disruptions are delaying surgeries and compromising patient care, survey finds
A patient safety nonprofit used the findings to call for “long-term, nationally coordinated solutions” to stop persistent shortages.
By Nick Paul Taylor • Oct. 19, 2023 -
Interventional Systems gets second 510(k) clearance for miniature robot
The FDA cleared the Micromate system to use CT navigation in procedures such as biopsies and ablations.
By Susan Kelly • Oct. 19, 2023 -
FDA finalizes COVID-era guidance, extending flexibility for some remote monitoring devices
The agency shortened its list of devices that can continue to undergo limited modifications without 510(k) filings.
By Nick Paul Taylor • Oct. 19, 2023 -
BD recycles 40,000 pounds of medical waste in circular economy pilot project
The collaborators assessed the feasibility of recycling medical waste such as plastic syringes.
By Nick Paul Taylor • Oct. 18, 2023 -
Abbott raises outlook amid strong Q3, sees momentum into 2024
Sales of the FreeStyle Libre continuous glucose monitor rose nearly 31% despite investor worries about the potential impact of weight-loss drugs on demand.
By Susan Kelly • Oct. 18, 2023 -
Siemens Healthineers partners to improve tuberculosis screening using AI
The company will work with the Global Fund to Fight AIDS, Tuberculosis and Malaria, providing free licenses and helping to integrate AI-enabled processes into clinicians’ workflows.
By Nick Paul Taylor • Oct. 18, 2023 -
J&J to restructure orthopedics business
The company announced the two-year restructuring in its third-quarter earnings call. It expects the changes to accelerate growth and enhance profitability, CFO Joe Wolk said.
By Elise Reuter • Oct. 17, 2023 -
Medtech can cope with GLP-1s but ‘fear and doubt’ suppressing stocks: analysts
J.P. Morgan analysts caution that stocks could continue to suffer through 2024 and 2025.
By Nick Paul Taylor • Oct. 17, 2023