Medical Devices: Page 45
-
J&J to phase out Janssen name in corporate rebrand
Janssen will be recast as Johnson & Johnson Innovative Medicine, while the company’s medical device business will remain Johnson & Johnson MedTech.
By Ned Pagliarulo • Sept. 15, 2023 -
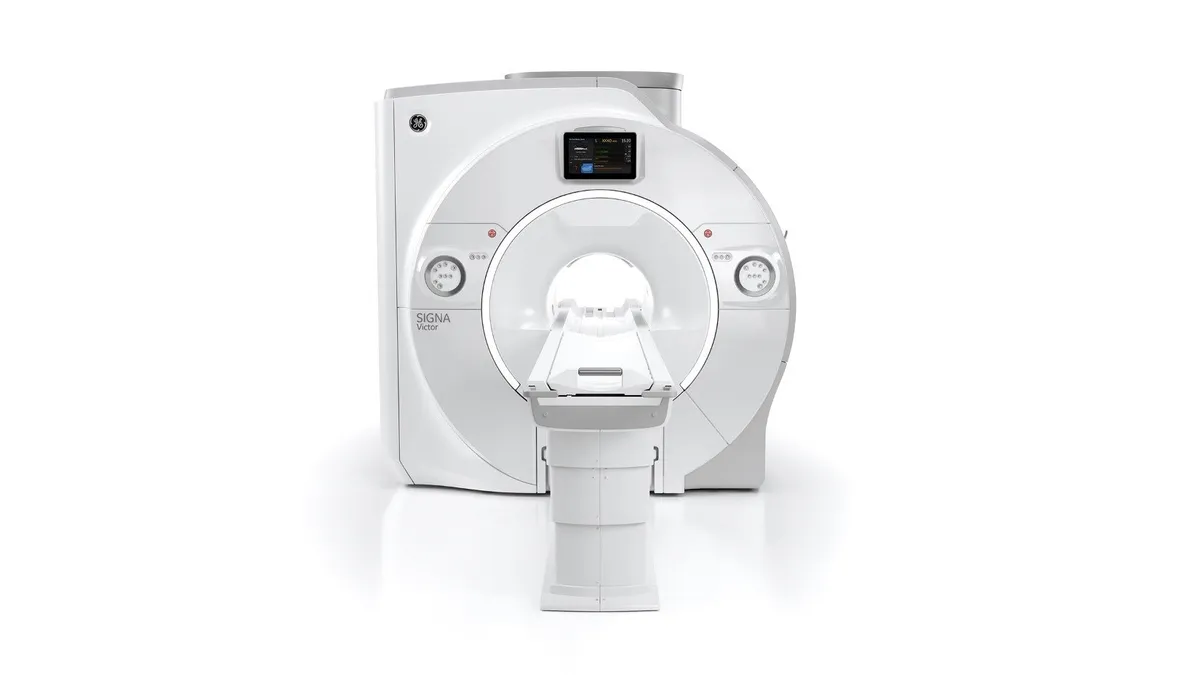
GE HealthCare partners with Mayo Clinic to accelerate work on imaging and AI
The organizations will collaborate on the application of AI to MRI and the automation of diagnostic and interventional ultrasound.
By Nick Paul Taylor • Sept. 15, 2023 -
Insulin pump-makers grapple with questions about GLP-1s
The new class of weight loss drugs has caused a stock selloff, but is unlikely to “meaningfully change the long-term outlook for diabetes,” analysts wrote.
By Elise Reuter • Sept. 14, 2023 -
FTC closes probe of Resonetics’ $900M medical nitinol purchase
The acquisition would give Resonetics a leading supplier of the alloy for the medical device industry.
By Elise Reuter • Sept. 14, 2023 -
Abbott’s neurostimulator recall, with 73 injuries reported, deemed Class I by FDA
The company contacted customers in July after receiving complaints from patients who were unable to exit the devices’ MRI mode.
By Nick Paul Taylor • Sept. 14, 2023 -
FDA finalizes biocompatibility guidance for devices that touch the skin
The agency chose not to make some major requested revisions, such as including metals in the scope of the guidance.
By Nick Paul Taylor • Sept. 14, 2023 -
Akili cuts 40% of workforce, plans shift to non-prescription model
The digital therapeutics company expects to extend its cash runway into 2025 with the changes.
By Elise Reuter • Sept. 13, 2023 -
Orthofix fires CEO, CFO after investigation finds misconduct
The executives engaged in “repeated inappropriate and offensive conduct” inconsistent with the company’s values, Orthofix said.
By Susan Kelly • Sept. 13, 2023 -
FDA finalizes combination product guidance 7 years after sharing draft
AdvaMed called overlapping human factor requirements of the draft guidance “overly burdensome.”
By Nick Paul Taylor • Sept. 13, 2023 -
TB deaths prompt FDA warning on reducing transmission risk
The agency outlined risk mitigation strategies for cell and tissue products after two recipients of bone matrix products died.
By Nick Paul Taylor • Sept. 13, 2023 -
CMS digs deeper into impact of proposed breakthrough device pathway in JAMA paper
The article discusses what the TCET pathway will mean for products at different stages of the life cycle.
By Nick Paul Taylor • Sept. 12, 2023 -
Bausch + Lomb seeks funding for $1.75B eye drop acquisition from Novartis
Fitch Ratings called the deal to buy the Xiidra treatment “strategically sound” even as the company’s plan to fund it would increase its leverage.
By Nick Paul Taylor • Sept. 12, 2023 -
Centinel Spine to sell fusion business, focus on disc replacement
Switzerland’s Silony Medical, which is buying the technology, sees itself as a challenger to “big medtech” in spinal fusion.
By Susan Kelly • Sept. 11, 2023 -
Q&A
Intuitive’s Tony Jarc on how AI is improving robotic surgery
The company aims to help surgeons achieve better results for patients and hospitals.
By Susan Kelly • Sept. 8, 2023 -
Sterigenics’ $408M ethylene oxide settlement removes ‘most problematic’ lawsuits: analysts
Remaining lawsuits pose less of a threat to the medical device sterilizer, and tighter emissions standards seem achievable, KeyBanc analysts wrote.
By Nick Paul Taylor • Sept. 8, 2023 -
PFA procedures expected to rise after Boston Scientific study results
Analysts estimate the technology could be used in up to half of all cardiac ablation cases in a few years.
By Elise Reuter • Sept. 8, 2023 -
Bayer faces Essure lawsuit after UK court clears 200 women to claim for damages
Bayer has vowed to defend itself “vigorously” against claims that could cost the company more than $12 million in damages.
By Nick Paul Taylor • Sept. 8, 2023 -
Zimmer Biomet promotes 3 executives
The appointments come less than a month after the company elevated Ivan Tornos to the CEO position.
By Susan Kelly • Sept. 7, 2023 -
Philips reaches settlement in US respiratory device litigation
The company will pay more than $479 million to resolve all economic loss claims. The settlement does not include injury or medical monitoring claims.
By Elise Reuter • Sept. 7, 2023 -
FDA proposes 3 guidances to improve 510(k) clearance process
The agency has made recommendations for selecting predicate devices, using clinical data and conducting performance testing for implants.
By Nick Paul Taylor • Sept. 7, 2023 -
Boston Scientific gets FDA nod for latest device in fast-growing Watchman franchise
Analysts at RBC Capital Markets called the Watchman FLX Pro approval an “incremental positive” for Boston Scientific.
By Nick Paul Taylor • Sept. 7, 2023 -
Synchron brain-computer interface implanted in first 6 US patients
The device is intended to give people with severe paralysis the ability to use their thoughts to perform everyday functions, such as online communications, hands-free.
By Susan Kelly • Sept. 6, 2023 -
Abbott to buy smart insulin pen cap maker Bigfoot
The planned acquisition could shift patients interested in smart pens to Abbott’s Libre CGMs, an analyst wrote.
By Elise Reuter • Sept. 6, 2023 -
Illumina names Agilent’s Jacob Thaysen as its next CEO
The appointment comes less than three months after former CEO Francis deSouza stepped down following a proxy battle.
By Susan Kelly • Sept. 5, 2023 -
Medtronic sued for allegedly sharing ‘treasure trove’ of diabetes patient data with Google
The plaintiff said disclosures to Google are “particularly problematic” because his use of Gmail meant his personal information was “automatically linked to his real identity.”
By Nick Paul Taylor • Sept. 5, 2023