FDA: Page 8
-
FDA authorizes Pi-Cardia’s valve-in-valve TAVR device
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.
By Nick Paul Taylor • Oct. 3, 2024 -
Siemens Healthineers wins FDA approval for 3D mammography system
The 3D capabilities are part of Siemens’ first completely redesigned mammography platform in over a decade.
By Nick Paul Taylor • Oct. 2, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
GE Healthcare gets FDA nod for new PET imaging agent
Called Flyrcado, the radiotracer could help improve cardiac imaging accuracy in patients with a high body mass index and women, the company said.
By Susan Kelly • Oct. 1, 2024 -
Establishment Labs wins FDA approval for Motiva breast implants
Motiva is the first new breast implant to receive premarket approval since 2013, according to Establishment Labs, and enters a market reshaped by safety issues linked to other products.
By Nick Paul Taylor • Sept. 30, 2024 -
Lawmakers call for investigation of former FDA device director
The letter to the health department watchdog follows a New York Times report that raised concerns about Jeff Shuren’s potential conflicts of interest as head of the FDA’s device center.
By Elise Reuter • Sept. 26, 2024 -
FDA proposes reclassifying hepatitis B assays
The FDA plans to move the assays from higher-risk class III devices to class II, making them eligible for the 510(k) pathway.
By Nick Paul Taylor • Sept. 26, 2024 -
FDA names new head of medical device evaluation and quality
Ross Segan was chosen to lead the office, which handles premarket authorizations and recalls, in the agency’s Center for Devices and Radiological Health.
By Susan Kelly • Sept. 24, 2024 -
FDA posts draft guidance on biocompatibility testing of devices
To promote consistency and reliability in premarket submissions, the FDA shared approaches for device biocompatibility analysis.
By Nick Paul Taylor • Sept. 23, 2024 -
GE Healthcare wins FDA clearance for Alzheimer’s imaging software
The clearance adds a Centiloid scale tool to GE Healthcare’s Mim software to help clinicians determine the density of amyloid plaque.
By Nick Paul Taylor • Sept. 20, 2024 -
Advamed backs FDA’s misinformation draft, calls for updates on AI and deep fakes
Advamed said the draft guidance “better reflects the wide scope of internet-based content seen in today’s information age” than an earlier version.
By Nick Paul Taylor • Sept. 16, 2024 -
Boston Scientific closes Silk Road Medical acquisition
Completion of the purchase comes three months after the companies announced the deal, and after Boston Scientific resubmitted its merger filing to give the FTC more review time.
By Ricky Zipp • Updated Sept. 17, 2024 -
Apple wins FDA nod for hearing aid software for certain Airpods
Apple is the first company to receive authorization for over-the-counter hearing aid software.
By Nick Paul Taylor • Sept. 13, 2024 -
FDA warns 2 Chinese labs for oversight failures, animal care violations
The FDA said the problems “raise concerns about the quality and integrity of data generated by the labs,” which provide third-party testing and validation data services for device firms.
By Nick Paul Taylor • Sept. 12, 2024 -
Dexcom, Abbott OTC glucose sensors add to busy year for diabetes tech
New over-the-counter sensors, an Abbott-Medtronic partnership and Roche’s first CGM are among diabetes technology’s top stories so far in 2024. Check out MedTech Dive’s roundup of the latest news.
By Ricky Zipp • Sept. 11, 2024 -
FDA to investigate presence of metals in tampons
Members of the Democratic Women’s Caucus last week urged FDA Commissioner Robert Califf to address the safety concerns.
By Nick Paul Taylor • Sept. 11, 2024 -
Lab group urges lawmakers to rescind FDA final rule on LDTs
A survey found 48% of labs will discontinue LDTs if they do not qualify for an exception under the FDA final rule.
By Nick Paul Taylor • Sept. 9, 2024 -

 Retrieved from The Food and Drug Administration.
Retrieved from The Food and Drug Administration.
FDA cracks down on ozone cleaners for CPAP machines
The agency sent warning letters to four companies and reminded the public it has not authorized any devices for cleaning or disinfecting CPAPs.
By Elise Reuter • Sept. 5, 2024 -
European heart group recommends renal denervation for some patients
The European Society of Cardiology said the treatment may be considered for certain patients with uncontrolled, drug-resistant high blood pressure but outlined lingering concerns.
By Nick Paul Taylor • Sept. 4, 2024 -
Illumina avoids fine for Grail purchase in European court victory
Illumina will avoid a penalty of 432 million euros after a court ruled the European Commission did not have jurisdiction to challenge the company’s Grail acquisition.
By Susan Kelly • Sept. 3, 2024 -
ARPA-H program to focus on AI degradation in medical tools
The agency will fund work to identify and auto-correct AI-enabled tools that are misaligned with their underlying training data.
By Nick Paul Taylor • Sept. 3, 2024 -
FDA finalizes Voluntary Malfunction Summary Reporting guidance
The agency clarified when companies can submit device malfunction reports in a quarterly summary, instead of individual reports every 30 days.
By Nick Paul Taylor • Aug. 30, 2024 -
Boston Scientific receives CE mark for updated TAVR technology
Changes include the addition of a larger valve size that company executives have said will open up 25% of the market in Europe.
By Nick Paul Taylor • Aug. 28, 2024 -
Illumina wins FDA approval for companion diagnostic cancer test
Physicians can use the test to identify people eligible for treatment with Bayer’s Vitrakvi and Eli Lilly’s Retevmo, which target cancers that have specific genetic features.
By Nick Paul Taylor • Aug. 28, 2024 -
FDA seeks feedback on predetermined change control plans
The guidance describes changes companies can make to medical devices without filing a new 510(k) or premarket approval supplement.
By Nick Paul Taylor • Aug. 26, 2024 -
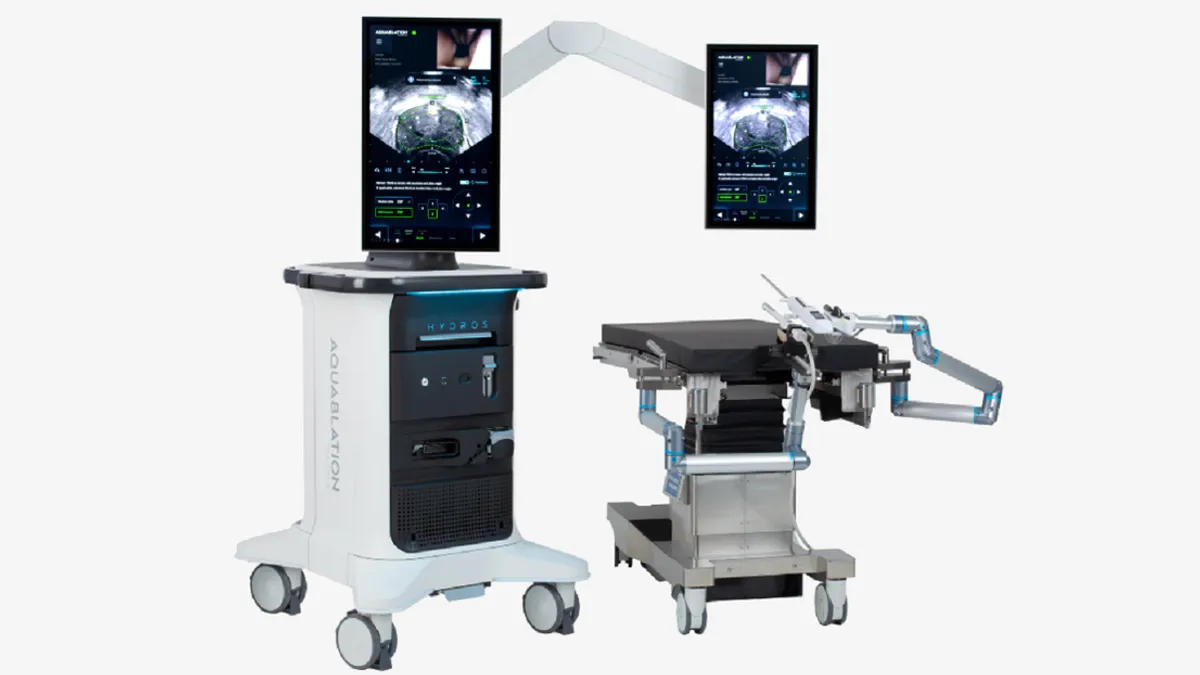
Procept wins FDA clearance for updated prostate surgery robot
Procept Biorobotics has added AI capabilities and switched to a single-use scope to support mass-market adoption.
By Nick Paul Taylor • Aug. 22, 2024