Recalls
-
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
By Nick Paul Taylor • Aug. 25, 2025 -
Medtronic recall of heart vent catheters tied to 3 serious injuries
The FDA said the request to quarantine the devices followed reports of the products “resisting shape retention when being bent.”
By Nick Paul Taylor • Aug. 19, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineMedical device safety in spotlight after high profile recalls
From Philips’ massive recall of respiratory devices to ongoing health risks with breast implants, medical devices tied to patient harm have put a focus on product safety.
By MedTech Dive staff -
Draeger removes ventilation filters over misleading carbon dioxide readings
Hospitals are being advised to stop using the filters after serious injuries were reported related to the problem, the FDA said in a recall notice.
By Elise Reuter • Aug. 12, 2025 -
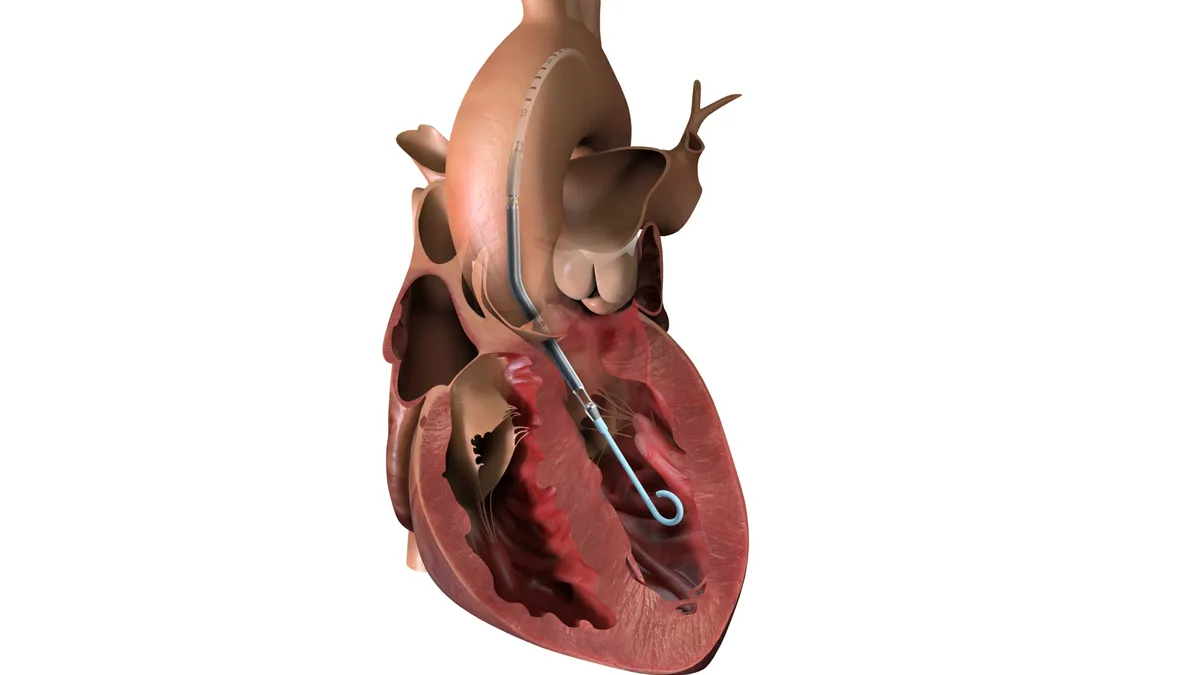
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
By Nick Paul Taylor • Aug. 8, 2025 -
Boston Scientific tells users about defibrillator problem linked to deaths, injuries
A company spokesperson said in a statement to MedTech Dive that direct causation between the deaths and calcification of the leads cannot be assumed or confirmed.
By Nick Paul Taylor • Aug. 7, 2025 -
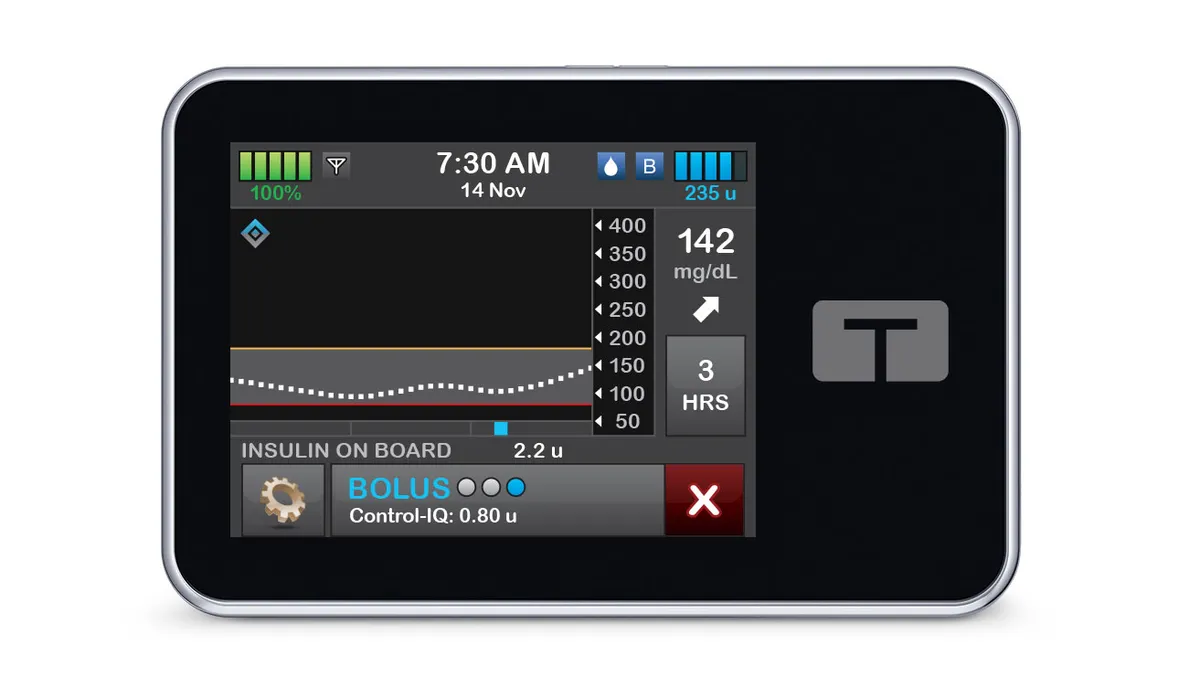
Tandem insulin pump malfunction linked to 59 injuries
A problem with speakers in Tandem’s t:slim X2 insulin pumps can cause insulin delivery to stop, the company said.
By Elise Reuter • Aug. 7, 2025 -
Philips BiPAP machine recall associated with 8 deaths
Philips sent updated instructions after disclosing problems last year with an alarm that can interrupt treatment for people with obstructive sleep apnea or respiratory insufficiency.
By Elise Reuter • Aug. 5, 2025 -
J&J’s Ethicon recalls stapler cartridges over issue linked to 1 death
The FDA said the device can lock, leading to adverse events including surgical delay, bleeding and death.
By Nick Paul Taylor • Updated July 29, 2025 -
Edwards recalls arterial cannulas over exposed wires
Exposed wires could puncture the artery and cause bleeding, inadequate perfusion and hemolysis, the FDA said in its Class I recall notice.
By Nick Paul Taylor • July 25, 2025 -
Baxter recalls certain Novum pumps over issues tied to 79 injuries, 2 deaths
The company has advised users to change the pump and infusion set at a time when a delay in treatment would not harm the patient.
By Nick Paul Taylor • July 23, 2025 -
Integra recalls cranial drills over defect linked to 10 injuries
The defect has resulted in problems including a procedural delay, difficulty removing device fragments and bleeding, the FDA said.
By Nick Paul Taylor • July 17, 2025 -
J&J’s Abiomed recalls heart pump controllers after 3 patients die
The FDA published an early alert, which the agency reserves for potentially high-risk issues.
By Nick Paul Taylor • Updated July 8, 2025 -
Cook Medical recalls catheters over fault linked to 3 serious injuries
The company began the recall after receiving four field complaints about tip separation before and during use.
By Nick Paul Taylor • June 27, 2025 -
Medtronic recall of capsule delivery devices tied to 33 serious injuries
The Food and Drug Administration published an early alert for the recall on Tuesday. No deaths have been associated with the problem.
By Ricky Zipp • June 25, 2025 -
GE Healthcare recalls Carestation devices over ventilation failure risk
The recall affects 15 Carestation models and more than 14,000 individual devices.
By Nick Paul Taylor • June 24, 2025 -
Q’Apel recalls clot removal device in response to FDA warning letter
Rather than pursuing a new regulatory pathway, Q'Apel said it is discontinuing the recalled system “as part of its strategic shift toward newer technologies.”
By Nick Paul Taylor • June 18, 2025 -
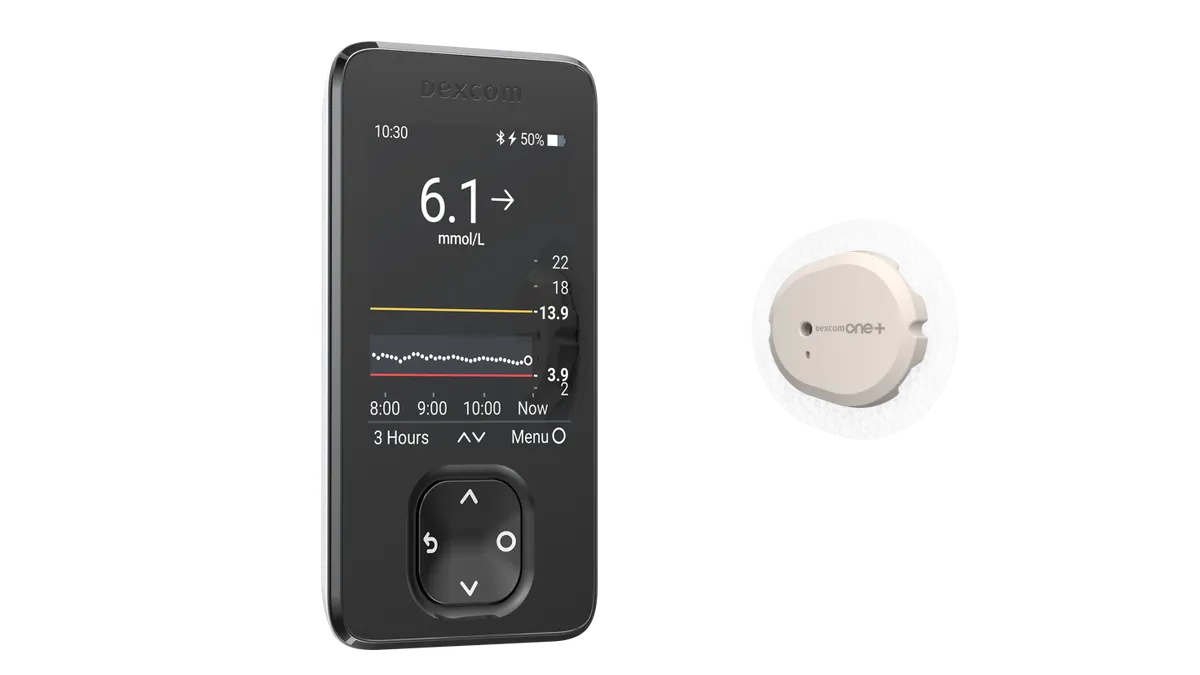
Dexcom recalls more than 700,000 CGM receivers for lack of audible alarm
Severe adverse events, including seizure and loss of consciousness, were potentially linked to the issue, based on 56 reports Dexcom received.
By Elise Reuter • Updated June 24, 2025 -
Zyno recalls infusion pumps over unvalidated software
Customers have been asked to stop using the pumps and wait for someone to contact them about exchanging devices.
By Nick Paul Taylor • June 17, 2025 -
Centerline recalls guidewire over risk of coating being left in patients
Coating for the guidewires could come off and get left in patients, which could result in extended or additional procedures. No serious injuries or deaths have been tied to the recall.
By Nick Paul Taylor • June 16, 2025 -
Medtronic recalls ventilators linked to 2 serious injuries, 1 death
The recall comes more than one year after Medtronic announced it would exit the ventilator market.
By Nick Paul Taylor • June 12, 2025 -
Baxter recalls Novum pump linked to 1 serious injury
The company has asked users to monitor patients frequently to ensure the appropriate infusion is being delivered.
By Nick Paul Taylor • Updated June 11, 2025 -
Medtronic removes tracheostomy tubes due to dislodging risk
If the tube moves out of place, it could prevent a patient from breathing or block the airway. A Medtronic spokesperson said patient harm was reported in some cases.
By Elise Reuter • June 6, 2025 -
Smiths Medical recalls infusion pumps over 3 problems
Smiths has not reported any serious injuries or deaths related to the issues, but the FDA sees a risk of harm.
By Nick Paul Taylor • June 4, 2025 -
BD recalls esophagogastric tubes linked to 2 serious injuries, 1 death
A problem when preparing the devices for use can delay diagnosis or treatment, leading BD to update its instructions.
By Nick Paul Taylor • May 27, 2025 -
HHS overhaul
RFK’s FDA layoffs could slow safety communications, experts warn
The mass cuts, which included communications staff, could slow public notices on medical device recalls and other safety alerts.
By Elise Reuter • May 6, 2025