FDA: Page 13
-
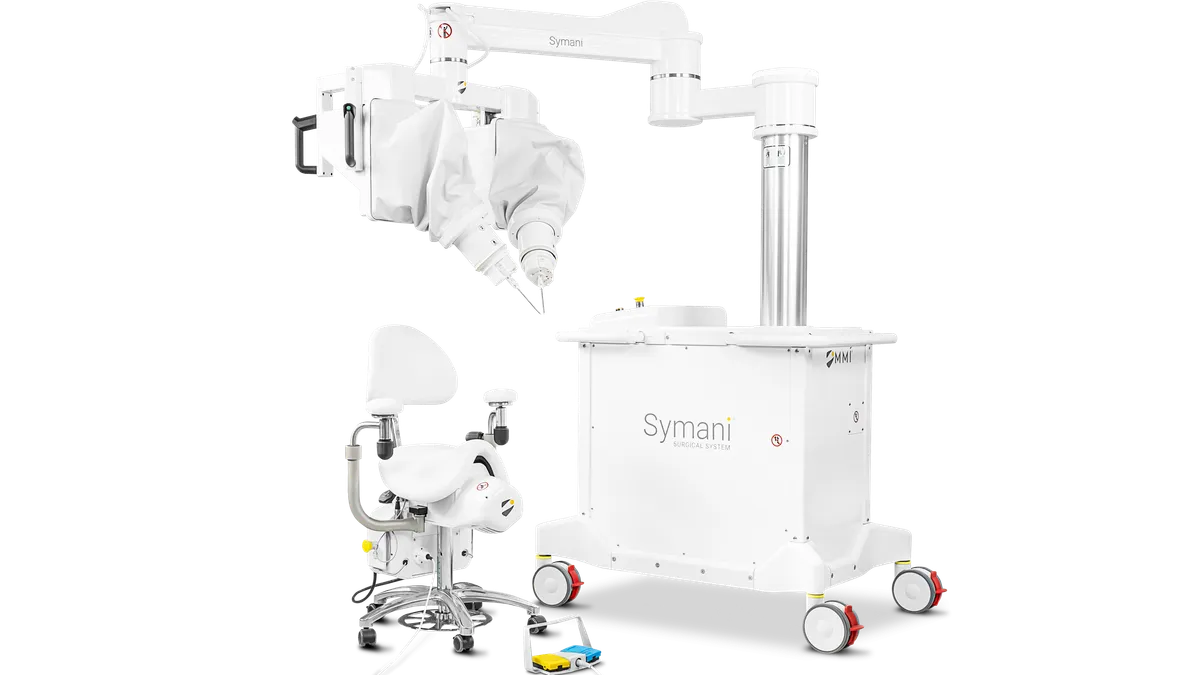
MMI receives de novo nod for microsurgery robot
Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.
By Nick Paul Taylor • April 9, 2024 -
Physicians ask FDA to revoke approval of DNA test for opioid addiction
Physicians said the test is based “on old genetic studies that have largely been abandoned” and could exacerbate the opioid crisis.
By Nick Paul Taylor • April 8, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Beckman Coulter receives FDA warning letter
Inspectors found fault with quality practices at a facility that makes immunoassay analyzer instruments and tests.
By Nick Paul Taylor • April 5, 2024 -
Smiths Medical recalls thousands of ventilators over fault linked to 8 serious injuries
A problem with the devices can cause patients to receive the wrong amount of ventilation or too little oxygen, the FDA said in a recall notice.
By Nick Paul Taylor • April 5, 2024 -
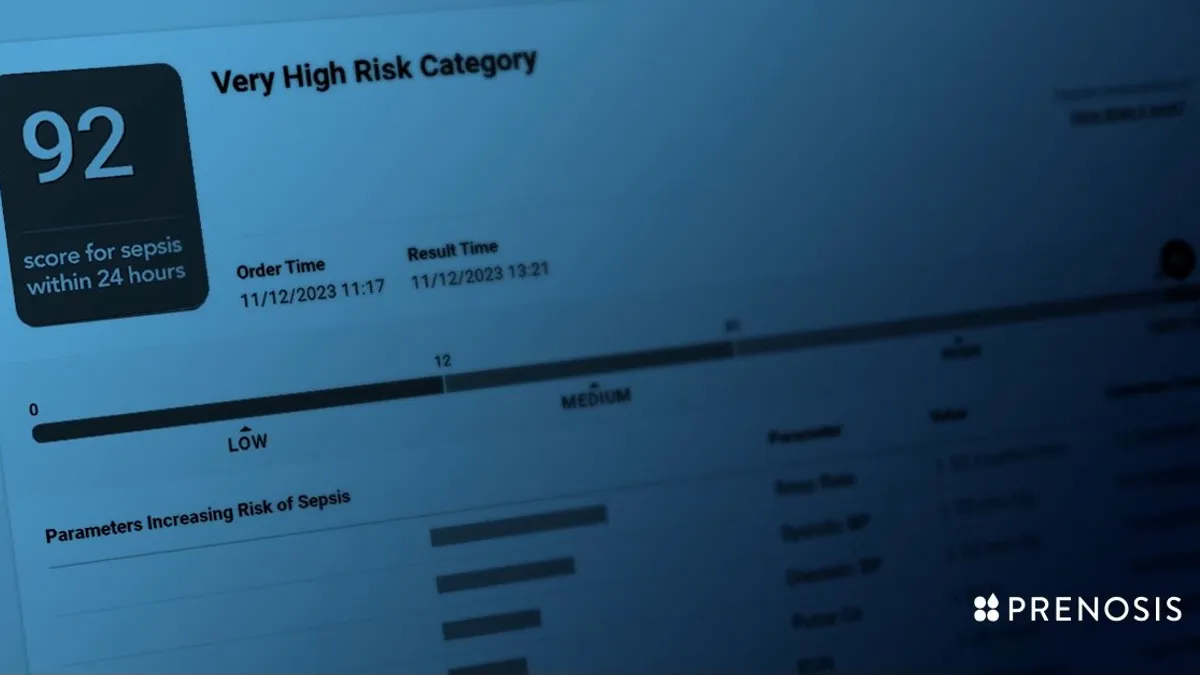
FDA grants de novo nod to AI tool for detecting sepsis
Prenosis CEO Bobby Reddy Jr. told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.
By Elise Reuter • April 4, 2024 -

 Retrieved from Axonics on August 22, 2023
Retrieved from Axonics on August 22, 2023
FTC asks Boston Scientific for more info on $3.7B Axonics deal
The company has pushed back its expected closing date for the acquisition due to the second request from the Federal Trade Commission.
By Susan Kelly • April 4, 2024 -
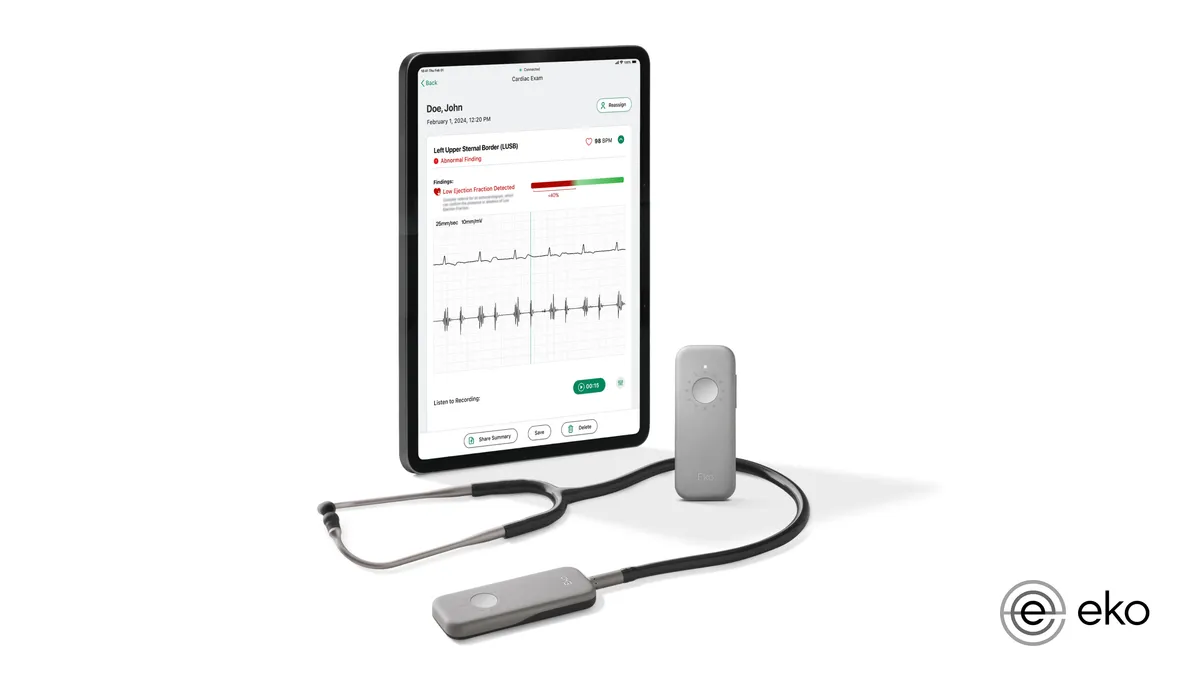
Eko wins FDA nod for AI to detect sign of heart failure using stethoscope
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
By Nick Paul Taylor • April 4, 2024 -
Teleflex catheterization kit recall linked to 10 injuries, 1 death
Nearly 335,000 devices were recalled due to safety issues, which may cause damage to blood vessel walls, artery blockage or death.
By Nick Paul Taylor • April 4, 2024 -
Deep Dive
As cyberattacks on healthcare persist, can the FDA’s new device regs hold up?
Revamped regulations to thwart hackers are a big step forward, but issues such as legacy devices and reliance on software patches pose lingering challenges.
By Ricky Zipp • April 3, 2024 -
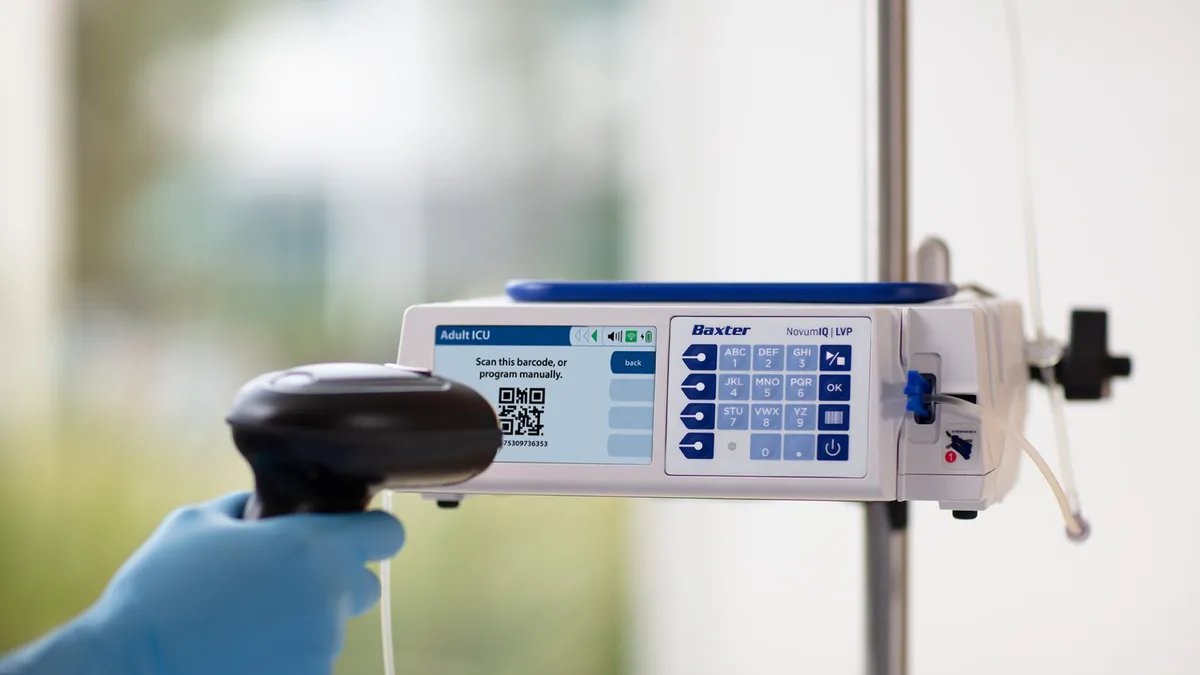
Baxter receives FDA clearance for delayed Novum IQ infusion pump
The clearance ends a three-year back-and-forth with the FDA to get the product to market.
By Nick Paul Taylor • April 2, 2024 -
Abbott wins FDA clearance for bedside blood concussion test
The clearance is a step towards using the test in non-healthcare settings, such as sporting events, Abbott said.
By Nick Paul Taylor • April 2, 2024 -
Infutronix pulls infusion pumps from US after nearly 3,700 complaints
The company listed six issues that can cause the pumps to stop infusions and otherwise malfunction.
By Nick Paul Taylor • April 2, 2024 -
Roche wins FDA approval for first molecular malaria blood donor screening test
The company is pitching the test as a way to improve the safety and availability of blood.
By Nick Paul Taylor • March 28, 2024 -
FDA hits Renovo with warning letter over reprocessed medical devices
The letter accuses Renovo of adding models other than the ones covered by its 510(k) clearances.
By Nick Paul Taylor • March 27, 2024 -
Neuronetics wins FDA clearance for device to treat adolescents with depression
William Blair analysts called the clearance a positive surprise, explaining that the FDA has denied several therapies in the patient population.
By Nick Paul Taylor • March 27, 2024 -
Abbott receives CE mark for 6-year insertable cardiac monitor
The device can continuously monitor a patient’s heart rhythms for either three or six years, depending on users’ needs.
By Nick Paul Taylor • March 26, 2024 -
J&J files for FDA approval of Varipulse pulsed field ablation platform
The company wants to catch up with rival PFA systems from Medtronic and Boston Scientific that have already received FDA authorization.
By Nick Paul Taylor • March 26, 2024 -
Vyaire Medical recalls Airlife resuscitators over defect linked to 2 death reports
The recall covers respiratory support devices made in 2017 or earlier that can fail to provide enough ventilation.
By Nick Paul Taylor • March 22, 2024 -
As FDA’s LDT rule looms, experts warn patients may lose access to critical tests
In testimony to Congress Thursday, industry and patient group leaders described the trade-offs expected from increased FDA oversight of laboratory-developed tests.
By Susan Kelly • March 22, 2024 -
Deka’s automated insulin delivery system, powered by patient-led app, gets FDA clearance
Sequel Med Tech will sell the new system, which integrates with Tidepool’s Loop insulin dosing algorithm.
By Nick Paul Taylor • March 20, 2024 -
FDA stops two manufacturers from importing plastic syringes
The agency issued import alerts to Jiangsu Caina Medical and Jiangsu Shenli Medical Production because their products didn’t meet quality requirements or were different from FDA-authorized versions.
By Nick Paul Taylor • Updated April 8, 2024 -
Deep Dive
6 ways the FDA can improve medical device recalls
Experts said improving how adverse events are tracked and requiring manufacturers to use electronic notifications could make devices safer.
By Elise Reuter • March 18, 2024 -
Fresenius Kabi receives FDA warning letter over issues at ex-Ivenix site
Inspectors found fault with the handling of corrective and preventive actions for the Ivenix Infusion System.
By Nick Paul Taylor • March 15, 2024 -
EPA final rule limits EtO emissions for medical device sterilizers
Medtech companies now have two years to come into compliance with the new regulations.
By Elise Reuter • March 14, 2024 -
Mass General Brigham works with FDA to create brain-computer interface group
The collaborators aim to resolve the clinical, regulatory, coverage and payment questions facing developers of the devices.
By Nick Paul Taylor • March 13, 2024