FDA: Page 14
-
FDA seeks feedback on expansion of premarket cybersecurity guidance
The agency is providing information on cybersecurity requirements for companies seeking authorization of new devices.
By Nick Paul Taylor • March 13, 2024 -
AI to expand medtech portfolios, revenue streams: Moody’s
The rating agency predicts AI will start to have a positive impact on medical device companies in the next two years.
By Nick Paul Taylor • March 12, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Deep Dive
Why Cigna is capping cost increases for pricey GLP-1 weight loss drugs
The first-of-its-kind move comes as pharmacy benefit managers continue to try to prove their value to clients, and shows how major players are shoring up to meet sky-high GLP-1 demand.
By Rebecca Pifer • March 11, 2024 -
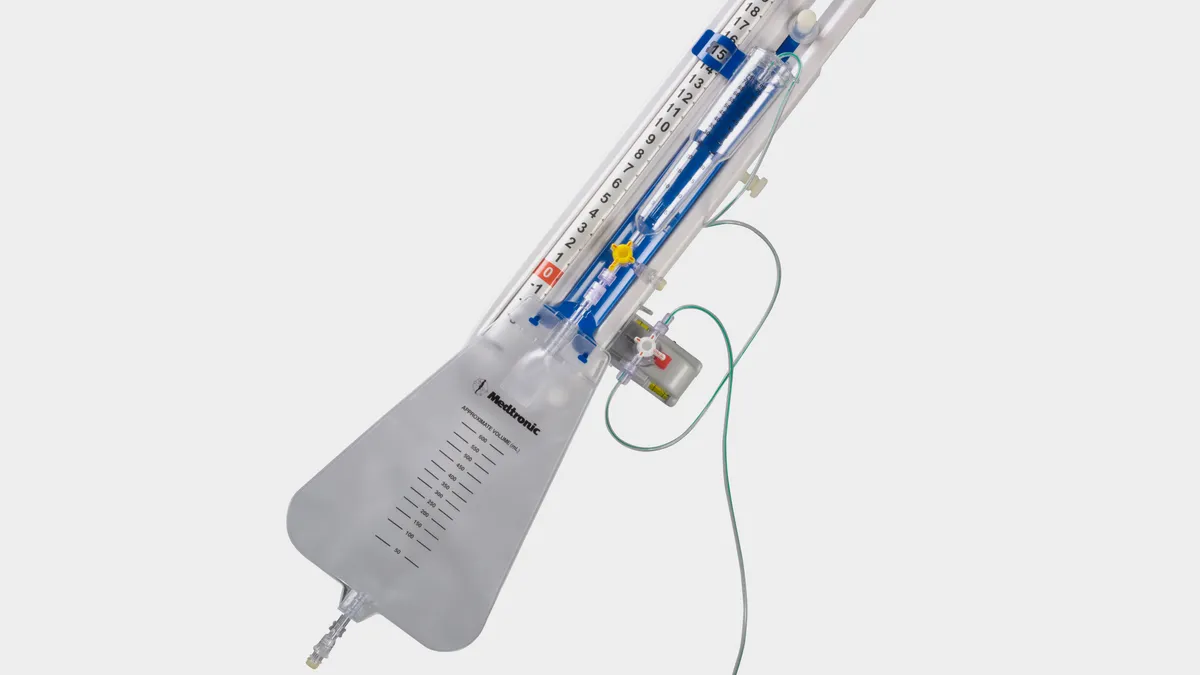
Medtronic recalls more than 45,000 catheter tubing units after injury reports
The issue, which is linked to 26 injuries, could result in neurological harm or death, FDA said.
By Nick Paul Taylor • March 8, 2024 -
Q&A
AI oversight is top challenge facing global device regulators: FDA official
Melissa Torres, CDRH’s associate director of international affairs, spoke about the importance of the International Medical Device Regulators Forum and how countries are struggling with AI oversight.
By Ricky Zipp • March 8, 2024 -
Dexcom receives FDA clearance for first OTC glucose sensor
The diabetes tech firm is tailoring its software to the 25 million people in the U.S. who have Type 2 diabetes and do not use insulin.
By Nick Paul Taylor • March 6, 2024 -
Dexcom, Novo Nordisk call for FDA input on digital diabetes detection devices
The companies want clarity on what evidence would be needed for new technologies to detect undiagnosed Type 2 diabetes or prediabetes.
By Nick Paul Taylor • March 4, 2024 -
GE Healthcare recalls incubators due to risk of newborns falling
The Food and Drug Administration labeled the recall as a Class I event after a serious injury was reported.
By Nick Paul Taylor • March 4, 2024 -
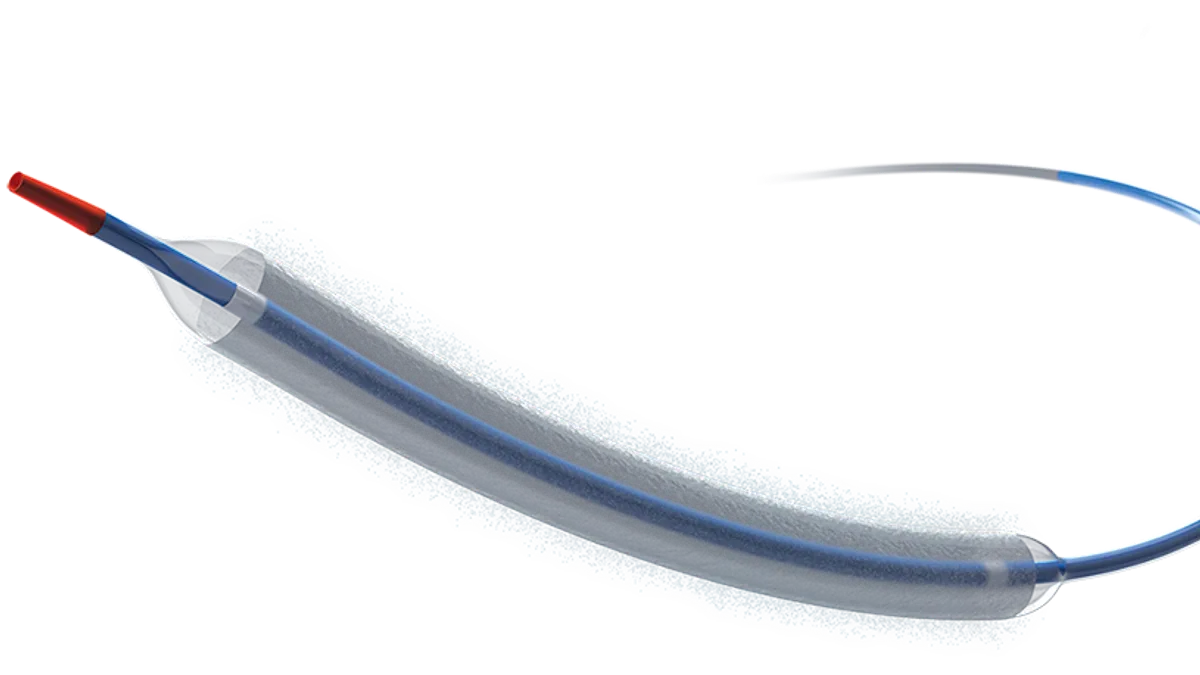
Boston Scientific gains FDA nod for drug-coated coronary balloon
BTIG analyst Marie Thibault said physicians could rapidly adopt the first-of-its-kind device.
By Susan Kelly • March 1, 2024 -
FDA responds to claim breast implant removal is ‘reasonable’ to cut cancer risk
Experts convened by the American Association of Plastic Surgeons concluded it may be “considered reasonable” to remove textured breast implants to reduce the risk of a rare cancer.
By Nick Paul Taylor • Updated March 4, 2024 -
J&J’s pulsed field ablation system secures European approval
The device maker will vie against rivals Boston Scientific and Medtronic in the market for the new treatment for atrial fibrillation.
By Susan Kelly • Feb. 29, 2024 -

 Retrieved from Hologic on February 28, 2024
Retrieved from Hologic on February 28, 2024
Hologic’s radiographic markers targeted in FDA safety communication
The agency has seen reports the implants can move out of position and break through the skin.
By Nick Paul Taylor • Feb. 28, 2024 -
Natera secures Medicare coverage for cancer test in 2 new indications
Natera met the coverage requirements for ovarian cancer in adjuvant and surveillance settings and for breast cancer in the neoadjuvant setting.
By Nick Paul Taylor • Feb. 27, 2024 -
Virtual Incision wins FDA de novo nod for miniaturized surgical robot
The device weighs about two pounds and fits into a tray that can be carried between operating rooms.
By Nick Paul Taylor • Feb. 27, 2024 -
European Council approves extension of IVDR transition
The Council expects the European Parliament to vote on the plan, without seeking amendments, in April.
By Nick Paul Taylor • Feb. 26, 2024 -
FDA warns against using smart wearables that claim to measure blood sugar
Unauthorized smartwatches and smart rings are “manufactured by dozens of companies and sold under multiple brand names,” according to the agency.
By Nick Paul Taylor • Feb. 23, 2024 -
Better Therapeutics wins breakthrough status for health app to treat liver disease
The FDA awarded the designation after seeing evidence the digital cognitive behavioral therapy may help reduce liver fat.
By Nick Paul Taylor • Feb. 21, 2024 -
CDRH’s Shuren backs expansion of TAP program to speed device approval
The FDA started the Total Product Lifecycle Advisory Program to help medtech companies cross the “valley of death” by getting agency feedback early.
By Elise Reuter • Feb. 21, 2024 -
FDA warns industry of ‘fraudulent’ third-party data in device applications
The agency has seen a rise in data that are fabricated, duplicated or otherwise unreliable in premarket submissions.
By Nick Paul Taylor • Feb. 21, 2024 -
Medical device recall system ‘failing to meet the needs of public health,’ physicians find
Amid a GAO review of recalls, the physicians advised the watchdog to revisit unique device identifiers and clarify the FDA’s enforcement authority.
By Nick Paul Taylor • Feb. 20, 2024 -
UK medical device reviewers form new association
The group intends to help the medical device sector navigate a post-Brexit regulatory landscape.
By Susan Kelly • Feb. 20, 2024 -
Top CDRH official to retire
William Maisel, director of the Office of Product Evaluation and Quality, will retire this spring, an FDA spokesperson confirmed.
By Elise Reuter • Feb. 15, 2024 -
Smiths Medical recalls syringe pumps for software malfunction
The FDA categorized the recall as Class I, noting that the software faults may cause pumps to fail, delaying or interrupting therapy.
By Nick Paul Taylor • Feb. 15, 2024 -
Masimo wins first FDA clearance for OTC medical fingertip pulse oximeter
The company's OTC device, which costs nearly $300, will compete with consumer pulse oximeters that are widely available but lack FDA clearance.
By Nick Paul Taylor • Feb. 14, 2024 -
FDA advisory panel overwhelmingly backs Abbott’s Triclip
The expert panel voted unanimously in favor of the safety of the tricuspid valve device, despite questions about a potential placebo effect in the trial.
By Ricky Zipp • Feb. 14, 2024