FDA: Page 18
-
FDA to establish digital health advisory committee
The agency is seeking advisers to help it understand the benefits, risks and outcomes associated with digital technologies.
By Elise Reuter • Oct. 12, 2023 -
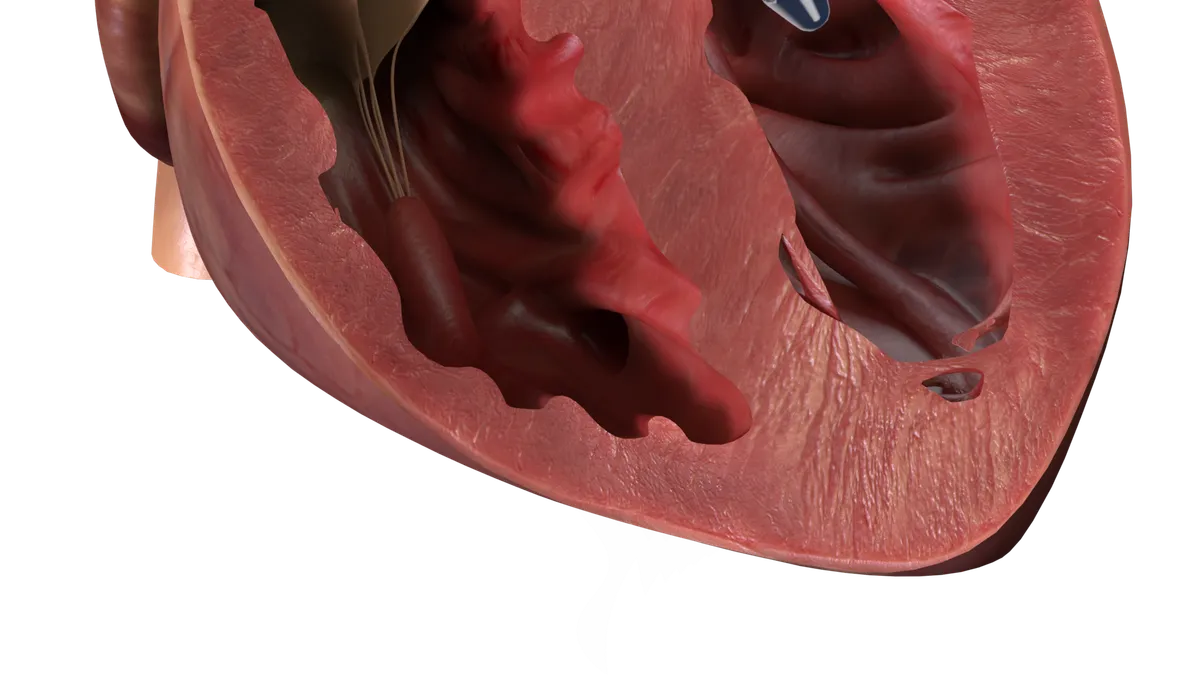
J&J’s Abiomed hit with FDA warning letter over Impella heart pump
The letter identifies quality system problems in a group of recalled devices and says monitoring software used with the pump requires premarket authorization.
By Susan Kelly • Oct. 12, 2023 -
Boston Scientific wins diabetic neuropathy approval, joining rivals in growing market
The expanded indication positions Boston Scientific to challenge Abbott, Medtronic and Nevro for the emerging opportunity in spinal cord stimulation to treat the condition.
By Nick Paul Taylor • Oct. 12, 2023 -
AdvaMed elevates digital health center to division, names board
The new division will focus on four core policy priorities under Chair Taha Kass-Hout, chief technology officer at GE HealthCare.
By Susan Kelly • Oct. 10, 2023 -
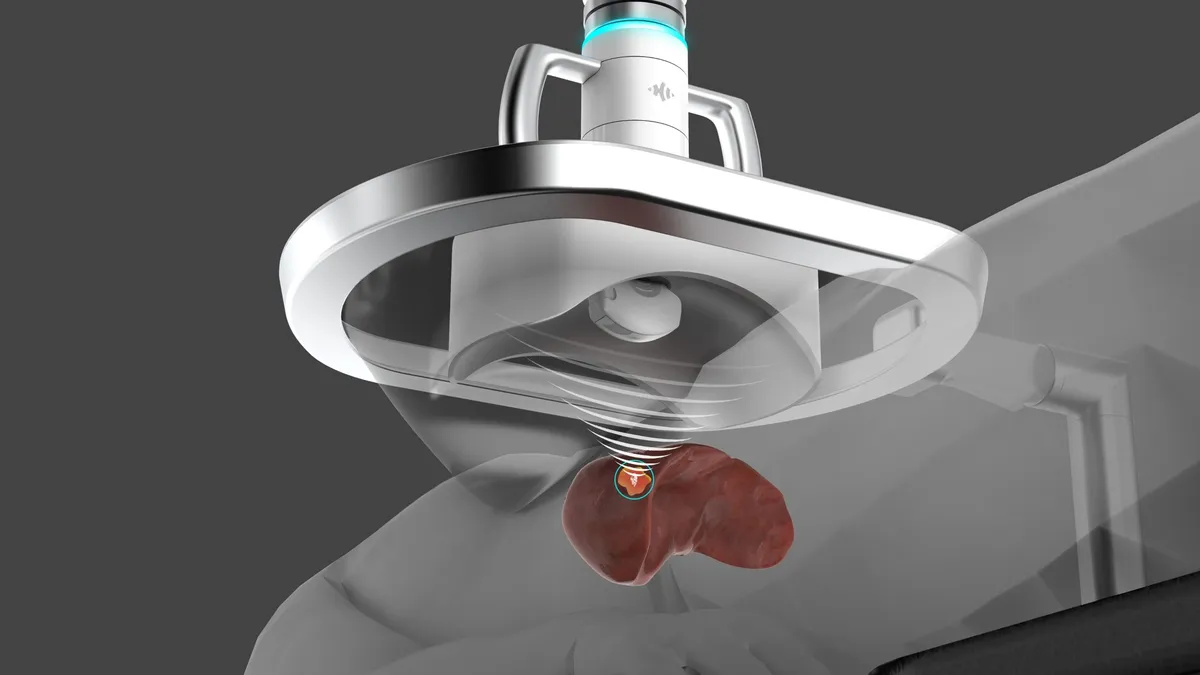
J&J-backed HistoSonics gets de novo clearance for liver cancer treatment
The system offers a minimally invasive alternative to surgical resection and thermal ablation in patients with primary and metastatic liver cancers.
By Nick Paul Taylor • Oct. 10, 2023 -
EU poised to mandate Illumina sell Grail liquid biopsy unit: FT
Regulators could order the divestment of the business as soon as next week to punish Illumina for defying their authority and “illegally” acquiring Grail.
By Nick Paul Taylor • Oct. 10, 2023 -
Invitae test for hereditary cancers gains FDA de novo nod
The test analyzes DNA from a single blood sample to identify hundreds of variants in 47 genes associated with predispositions for a range of cancer types.
By Susan Kelly • Oct. 3, 2023 -
FDA expands total product life cycle program to cover neurological devices
The agency could enroll up to 45 additional devices over the coming year.
By Nick Paul Taylor • Oct. 3, 2023 -
FDA details switch to electronic filings for 510(k), de novo submissions
The guidance documents advance the transition to eSTAR, an electronic template the FDA is adopting to meet legislative requirements.
By Nick Paul Taylor • Oct. 3, 2023 -
Mayo Clinic partner earns 510(k) clearance for heart failure screening algorithm
The algorithm analyzes 12-lead ECGs to show if a patient may be developing heart failure.
By Nick Paul Taylor • Oct. 2, 2023 -
FDA to regulate lab-developed tests under proposed rule
The tests have come under scrutiny by the agency and lawmakers, but the FDA is still likely to face pushback on the changes.
By Elise Reuter • Sept. 29, 2023 -
FDA posts guidance on how to simplify updates to antimicrobial resistance tests
The advice focuses on predetermined change control plans, a mechanism the regulator sees as a way to streamline updates in multiple areas.
By Nick Paul Taylor • Sept. 29, 2023 -
As government shutdown looms, medical device reviews expected to continue
The FDA could furlough nearly a fifth of its staff by Sunday if Congress doesn’t reach a funding agreement.
By Elise Reuter • Sept. 28, 2023 -
FDA finalizes guidance on cybersecurity for medical devices
Congress granted the agency authority to deny premarket submissions that lack cybersecurity information, starting in October.
By Nick Paul Taylor • Sept. 27, 2023 -
Edwards Lifesciences says it was target of surprise EU antitrust inspection
The European Commission announced the action last week without naming the cardiovascular device company.
By Susan Kelly • Sept. 26, 2023 -
Intarcia’s diabetes drug-device combo voted down again by FDA panel
The 19-member advisory committee unanimously voted against Intarcia in the review, citing cardiovascular and kidney safety concerns.
By Gwendolyn Wu • Sept. 25, 2023 -
Medtronic receives CE mark for CGM sensor, boosting challenge to Abbott and Dexcom
Medtronic has made the sensor smaller and eliminated the need for fingersticks to calibrate the device, bringing a challenger to market leaders Abbott and Dexcom.
By Nick Paul Taylor • Sept. 25, 2023 -
FDA shares draft medical device harmonization plan to meet MDUFA V goal
The FDA outlined deadlines for actions it will take, including creating a mechanism for working with regulatory partners.
By Nick Paul Taylor • Sept. 25, 2023 -

 "Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
"Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
Limited resources constrain federal oversight of medical device ads, GAO finds
U.S. agencies took 322 enforcement actions related to medtech advertising between 2018 and 2022.
By Nick Paul Taylor • Sept. 21, 2023 -
EU antitrust officials make surprise inspections at cardiovascular device company
The unnamed company may have violated EU rules against abusing a dominant market position, the European Commission said.
By Susan Kelly • Sept. 20, 2023 -
CMS leader defends breakthrough device reimbursement proposal at House hearing
Dora Hughes made the case for restricting the Transitional Coverage for Emerging Technologies pathway to five reviews a year.
By Nick Paul Taylor • Sept. 20, 2023 -
FDA makes device conformity testing scheme permanent to streamline assessments
The FDA has delivered on a MDUFA V commitment by turning ASCA into a permanent, voluntary accreditation program.
By Nick Paul Taylor • Sept. 20, 2023 -
FDA releases draft guidance for studying weight loss devices
The documents arrive amid questions about whether new medicines will cut demand for weight loss procedures.
By Nick Paul Taylor • Sept. 19, 2023 -
MedTech Europe says MDR, IVDR framework needs ‘structural reform’
Patients may face delayed access to treatments unless changes are made to the new regulatory system, 35 European trade groups warned in an open letter.
By Susan Kelly • Sept. 18, 2023 -
FDA finalizes breakthrough device changes to target health inequities
The proposals received positive feedback from groups including AdvaMed, leading the FDA to finalize the changes with only minor revisions.
By Nick Paul Taylor • Sept. 18, 2023