FDA: Page 19
-
Beacon Biosignals receives FDA clearance for sleep tracking headband
The company’s at-home device, the Dreem 3S, has six EEG electrodes to capture brain activity.
By Nick Paul Taylor • Sept. 18, 2023 -
Abbott’s neurostimulator recall, with 73 injuries reported, deemed Class I by FDA
The company contacted customers in July after receiving complaints from patients who were unable to exit the devices’ MRI mode.
By Nick Paul Taylor • Sept. 14, 2023 -
FDA finalizes biocompatibility guidance for devices that touch the skin
The agency chose not to make some major requested revisions, such as including metals in the scope of the guidance.
By Nick Paul Taylor • Sept. 14, 2023 -
FDA finalizes combination product guidance 7 years after sharing draft
AdvaMed called overlapping human factor requirements of the draft guidance “overly burdensome.”
By Nick Paul Taylor • Sept. 13, 2023 -
TB deaths prompt FDA warning on reducing transmission risk
The agency outlined risk mitigation strategies for cell and tissue products after two recipients of bone matrix products died.
By Nick Paul Taylor • Sept. 13, 2023 -
CMS digs deeper into impact of proposed breakthrough device pathway in JAMA paper
The article discusses what the TCET pathway will mean for products at different stages of the life cycle.
By Nick Paul Taylor • Sept. 12, 2023 -
FDA proposes 3 guidances to improve 510(k) clearance process
The agency has made recommendations for selecting predicate devices, using clinical data and conducting performance testing for implants.
By Nick Paul Taylor • Sept. 7, 2023 -
Boston Scientific gets FDA nod for latest device in fast-growing Watchman franchise
Analysts at RBC Capital Markets called the Watchman FLX Pro approval an “incremental positive” for Boston Scientific.
By Nick Paul Taylor • Sept. 7, 2023 -
23andMe wins FDA clearance to test for expanded set of BRCA cancer risk variants
The new version of the test can report more cancer-related gene mutations, and 23andMe can add variants in the future under an agreement with the FDA.
By Nick Paul Taylor • Sept. 5, 2023 -
UK regulators name 3 approved bodies to ease device certification bottleneck
A MHRA leader hailed the action as “almost doubling capacity for medical device assessment in the U.K.”
By Nick Paul Taylor • Aug. 31, 2023 -
Hamilton Medical’s urgent ventilator notice deemed Class I recall by FDA
The company contacted customers in June after learning that long-term use of the devices could cause them to stop providing active ventilation.
By Nick Paul Taylor • Aug. 31, 2023 -
Trade groups urge CMS to expand, quickly finalize breakthrough device pathway
The groups want changes that include expanding the program to cover breakthrough diagnostics and previously authorized products.
By Nick Paul Taylor • Aug. 30, 2023 -
Withings wins FDA clearance for scale with AFib detection
The device records the electrical signal from the heart, assesses the number of calories the body burns at rest and tracks other health variables.
By Nick Paul Taylor • Aug. 30, 2023 -


Photo by Anna Tarazevich from Pexels

Hopes dim for Medtronic hypertension treatment after FDA panel rebuff
Analysts foresee hurdles to reimbursement even if the device wins the agency’s nod, and some don’t expect approval at all.
By Susan Kelly • Aug. 29, 2023 -
US signs consent decree requiring EPA to finalize ethylene oxide rule by March
A medtech trade group called the deadline inadequate for the EPA to complete the “substantial” work that remains.
By Nick Paul Taylor • Aug. 28, 2023 -
Medtronic renal denervation device hits setback at FDA panel
The agency’s outside advisers found the risks of the procedure to lower blood pressure outweighed the benefits in a narrow vote.
By Susan Kelly • Aug. 24, 2023 -
Draeger’s ventilator sound insulation recall triggers FDA Class I notification
The company found concentrations of a potentially carcinogenic foam component were above acceptable levels for children.
By Nick Paul Taylor • Aug. 24, 2023 -
Recor wins FDA panel’s backing for high blood pressure device
The advisers found the company’s renal denervation system is safe and effective, but several panelists expressed concern about the long-term durability of the treatment.
By Susan Kelly • Aug. 23, 2023 -
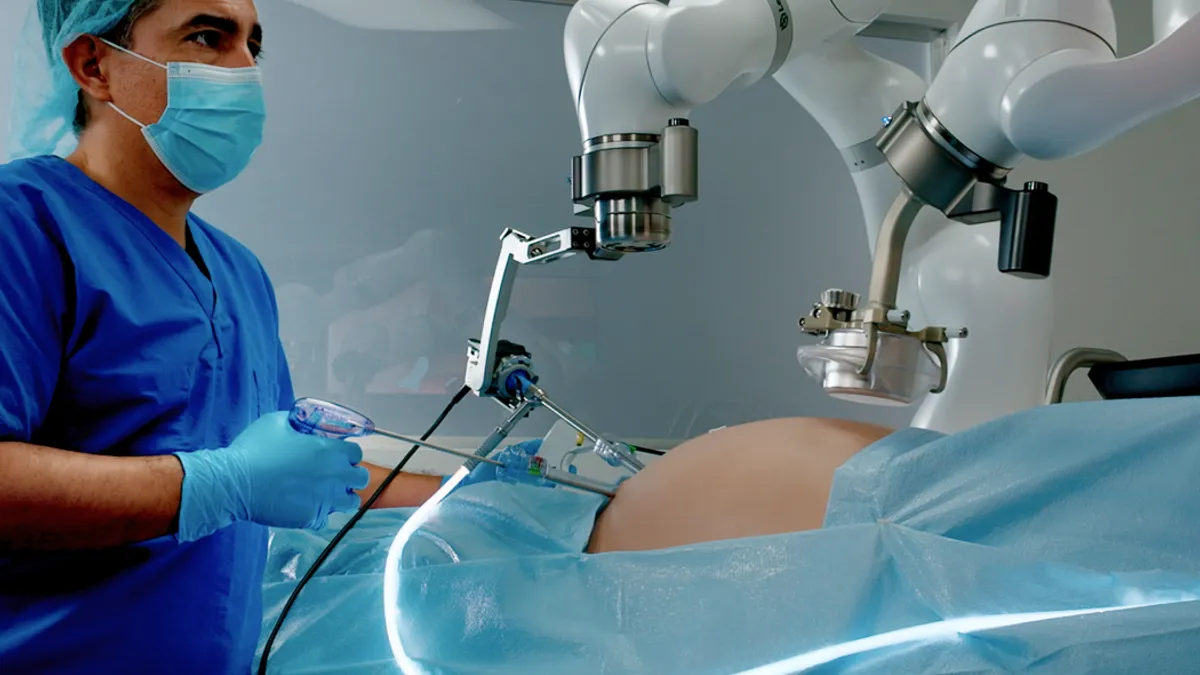
Levita’s surgeon-controlled arm for magnetic procedures gains 510(k) clearance
The device maker says the technology can reduce the need for an additional assistant in the operating room, driving efficiency.
By Nick Paul Taylor • Aug. 23, 2023 -

 Retrieved from GE HealthCare via Business Wire.
Retrieved from GE HealthCare via Business Wire.
GE HealthCare wins FDA clearance for wireless hospital vital sign monitor
The Portrait Mobile device is one of GE HealthCare’s “first major introductions in monitoring in recent years,” according to the company.
By Nick Paul Taylor • Aug. 16, 2023 -
Illumina faces SEC probe over Grail acquisition
The securities regulator has asked for documents tied to the 2021 deal in addition to the conduct and compensation of members of Illumina and Grail management.
By Susan Kelly • Aug. 14, 2023 -
Median lag between FDA authorization and Medicare coverage hits 6 years: study
Fewer than 30% of novel medical devices obtain some Medicare coverage in the three years after regulatory authorization, researchers found.
By Nick Paul Taylor • Aug. 11, 2023 -
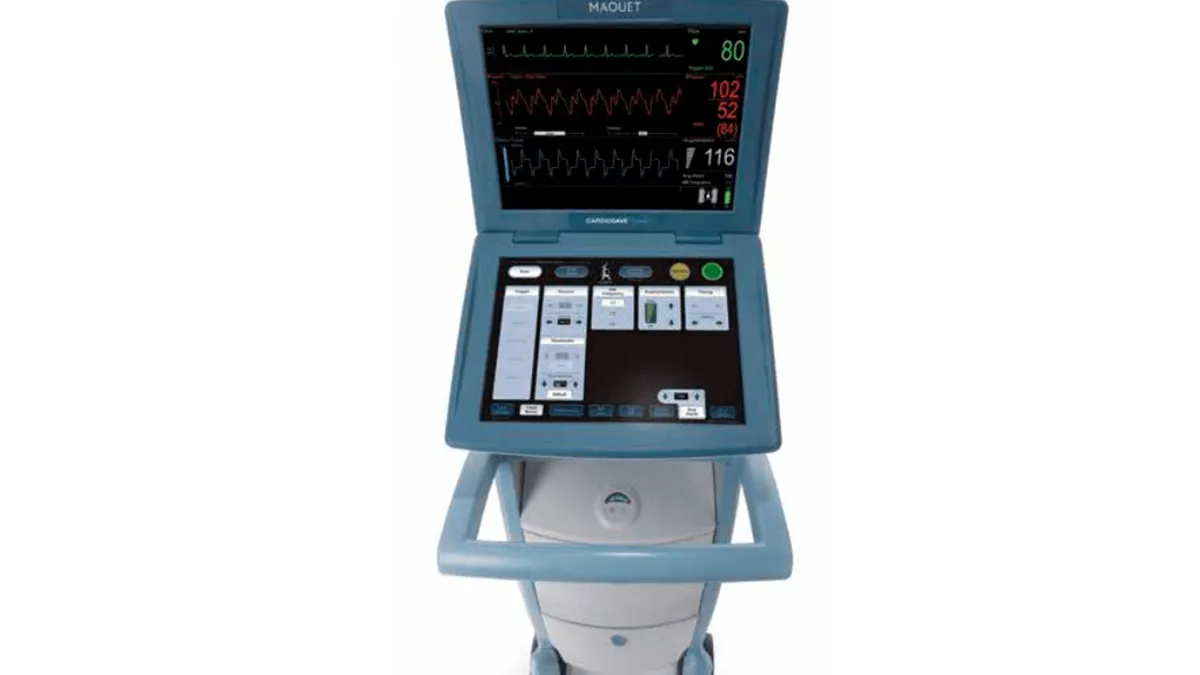
Getinge’s heart assist pump shutdown problem given FDA Class I recall tag
The company notified healthcare providers after receiving 26 complaints about Cardiosave devices, used to support severely ill patients, unexpectedly shutting down.
By Nick Paul Taylor • Aug. 11, 2023 -
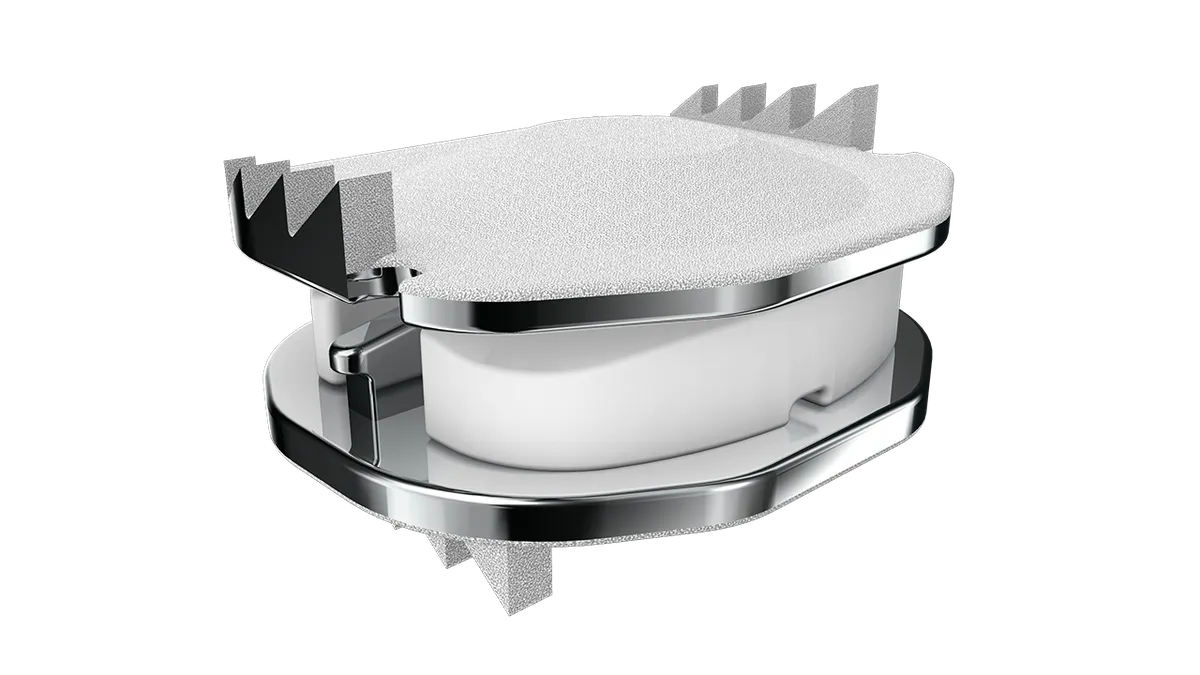
ZimVie wins FDA approval for smaller Mobi-C cervical disc
The device maker said the new product will “address the anatomical needs of the U.S. patient population.”
By Nick Paul Taylor • Aug. 10, 2023 -
Abbott lands FDA clearance of Alinity complete blood count system
The company claims the device uses less floor space and has one of the highest throughputs as it competes with diagnostic systems made by Beckman Coulter and Siemens Healthineers.
By Nick Paul Taylor • Aug. 8, 2023